New Therapy Award Recipients
The mission of the Epilepsy Therapy Project of the Epilepsy Foundation is to accelerate ideas into new therapies for people living with epilepsy and seizures.
In May 2004, the Epilepsy Therapy Project announced its inaugural translational research grant recipient awards.These grants are unique in that they were commercialization grants focusing on projects demonstrating a clear path from research in the laboratory to new treatments for epilepsy. The Epilepsy Foundation joined forces with the Epilepsy Therapy Project to support this venture and fund new, innovative translational research to speed the search for new therapies and a cure for epilepsy. In 2013, the Epilepsy Therapy Project merged with the Epilepsy Foundation. We at the foundation are proud to keep this research initiative going. Our New Therapy Commercialization Grants Program solicits grant proposals biannually.
We have proudly supported the commercialization of research originating either in the private or the academic sector in order to facilitate the development of new treatments. The Epilepsy Therapy Project has made and continues to make investments in promising start-up companies with an emphasis on finding new treatments for epilepsy and assists in finding additional sources of funding for such companies.
The Epilepsy Therapy Project is pleased to present the recipients of funds awarded to date.
Fall 2018: New Therapy Grant Award
- Project Title: Val-1221 to treat Lafora Disease
- Project Title: Biomarker Targeted Stimulation for Epilepsy: A Multicenter Series
Fall 2017: New Therapy Grant Award
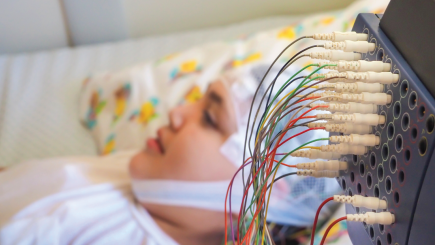
- Project Title: Routine EEG made simpler, faster and available anywhere
- Dry electrode headset providing medical grade EEG
- No need for skin preparation or gel/paste
- Comfortable and convenient to wear
- Easy size adjustments that fit children and adult head sizes and most hair types
- Accurate 10-20 EEG placement
- Designed for re-use and easy cleanability
- Snap on/Snap off electrodes (different heights and hot swappable) for good electrode contact for various head sizes and hair types
- Seamless data sharing and interpretation
This award is an investment from the Foundation to help Zeto commercialize and launch their first prototypes onto the marketplace.
Spring 2017: New Therapy Grant Award
- Project Title: Anti-seizure and -epileptogenic effects of new, selective mTOR inhibitors
Dr. Löscher has recently partnered with PIQUR Therapeutics, a company which developed a new class of mTOR inhibitor compounds. These next generation mTOR inhibitor drugs have a better safety profile than evorolimus. With this grant, Dr. Löscher will test their lead compounds on a battery of animal models to assess the compounds effectiveness at reducing seizures. If the data are promising, these studies will be the preliminary evidence needed to start a small clinical trial in Tuberous Sclerosis patients within 24 months.
Several studies indicate that the mTOR signaling pathway is also overactive in other epilepsy conditions. Interestingly, some of these studies also suggested mTOR inhibitor treatment following an acquired brain injury (such as a stroke or head trauma) could prevent epilepsy. Therefore, Dr. Löscher will also test whether these compounds could prevent the development of post-traumatic epilepsy in animal models with induced epilepsy. The work from this grant will be used to demonstrate proof of concept of these compounds as a treatment for epilepsy beyond Tuberous Sclerosis.
Fall 2016: New Therapy Grant Award
- Project Title: Ataluren for non-sense mutation in CDKL5 and Dravet Syndrome
Spring 2016: New Therapy Grant Award
- Project Title: EEG Patch | Non-Invasive Seizure Counting
Fall 2015: New Therapy Grant Award
- Project Title: Prototype of a Portable LSPR Device for PoC Measurement of AED Blood Levels
Spring 2015: New Therapy Grant Award
-
Project Title: Safety of the Hovding Inflatable Helmet in Seizures
Fall 2014: New Therapy Grant Award
-
Project Title: Disposable Chips to Measure Antiepileptic Drug Serum Concentrations at POC
Utkan Demirci, PhD
Stanford University
Palo Alto, CA
Summary: Epilepsy is a neurological disorder, and nearly one in three patients with epilepsy report dose-related side-effects from their seizure medication(s). These effects can be minimized by adjusting the antiepileptic drug (AED) dosage and timing of the dosage. Here, we propose to develop a disposable AED detection system that can be performed anywhere and automated to handle a drop of blood obtained with a finger prick, can give results in approximately 10 minutes.
Read More...

Utkan Demirci, Ph.D., is an Associate Professor at Stanford University School of Medicine, Canary Center at Stanford for Cancer Early Detection. He received his bachelor's degree in Electrical Engineering (Summa Cum Laude) in 1999 from the University of Michigan, Ann Arbor, and his master's degrees in Electrical Engineering in 2001 and in Management Science and Engineering in 2005 from Stanford University, and his doctorate in Electrical Engineering in 2005, also from Stanford University. His current work involves applying nano- and micro-scale technologies to manipulate cells in nano-liter volumes, with applications in infectious disease diagnostics and monitoring, cell encapsulation and assembly for cryobiology, tissue engineering, and regenerative medicine. His research interests include the applications of microelectromechanical systems (MEMS) and acoustics in medicine. The importance of his work to the field of point-of-care (POC) diagnostics for HIV is exemplified by his being awarded the NSF CAREER Award, IEEE EMBS Early Career Award, MIT TR-35 award, Global JCI Medical innovator award, Young Investigator Award from Coulter Foundation, and Department of Medicine Young Investigator Award. The honors and recognition he has received prove his successful track record in applying forefront technologies to problems in the clinic focusing on medical diagnostics and disposable microchips for the POC and bedside applications. He has also translated two start-up companies over the past 3 years that use technologies developed in his laboratory. DxNow Inc. in Boston develops POC tools for detecting infections early for dialysis patients. Koek Biotech is founded in Izmir and has products that are in clinical trials to increase fertility rates at In Vitro Fertilization (IVF) Clinics.
Dr. Demirci has also mentored over 30 post-doctoral fellows, 6 graduate students, over 60 undergraduate students, 25 graduate level visiting students/technicians over the past 7 years, who have continued to successful careers. A significant portion of the postdoctoral fellows trained in his laboratory continued successful academic careers in the US and globally at distinguished institutions. Only in 2014 eight postdoctoral trainees from his lab joined academic ranks as faculty. They hold academic positions at prestigious institutions globally such as Harvard Medical School, Case Western, UConn, Florida Atlantic University, Zhejiang University, Xi’an Jiatong University, Izmir Institute of Technology. Further, Dr. Demirci has recognized the importance of communication among the members of a project and hold weekly team meetings, where he is closely involved. He has been successful in encouraging frequent communication between project members within his own laboratory, with other labs on campus and other institutions. This approach has led to high profile peer-reviewed publications. He has authored over 80 peer-reviewed journal publications in journals, including Nature Communications, Nature Materials, PNAS, Advanced Materials, and Lab on a Chip, more than 100 conference abstracts and proceedings, and 12 book chapters and has edited a book on POC diagnostics. His work has been highlighted in Wired magazine, Nature Photonics, Nature Medicine, MIT Technology Review magazine, AIP News, BioTechniques, and Biophotonics. He has given over 100 national and international presentations, including invited keynotes at various academic, governmental, and industrial institutions. His work has been cited 1,500 times within the last two years with a citation H factor of 32.
Fall 2014: Epilepsy Seal of Excellence Award
- Project Title: Brain Implantable Focal Cooling and Sensing Array
Hitten Zaveri, PhD
Yale University
New Haven, CT
Summary: We seek to develop a brain implantable array of sensors and cooling elements. This array will allow the continuous measurement of brain activity and on demand controlled cooling of the cortex to stop seizures.
Read More...

Dr. Zaveri has received academic training in electrical engineering (B.S.E, M.S.E), computer engineering (B.S.E) and biomedical engineering (M.S., Ph.D.) from the University of Michigan in Ann Arbor and epilepsy and neurology (post-doctoral studies) from Yale University. He is the director of the Computational Neurophysiology Laboratory at Yale University. His research interests lie at the intersection of neuroscience, engineering and mathematics.
September 2014: Indiegogo Campaign
-
Innovation-A-Go-Go Grant: SAMi – Sleep Activity Monitor
HiPass Design - Charles and Cynthia Anderson
Colorado
Summary: There are over 37,000 children diagnosed with RS (Recurring Seizures) every year with an estimated total of 50,000,000 people with RS worldwide. Many caregivers either do not know when seizures occur during the night or they lose a lot of sleep trying to stay awake in case a seizure occurs. SAMi™ is a sleep activity monitor for caregivers who need to know when unusual movements occur during the night.
Read More...

Charles is the technical lead on project SAMi. Charles has a BS degree in Electrical Engineering and Computer Science from Tufts University. With 35 years of experience in hardware and software engineering Charles also has a proven track record in building and leading engineering teams.
Spring 2014: New Therapy Grant Awards
-
Project Title: Prodrug / Enzyme Systems for Intranasal Treatment of Seizure Emergencies
James Cloyd, PharmD
University of Minnesota
Minneapolis, Minnesota
Summary: Seizure emergencies can result in injury, increased medical costs and, if left untreated, can progress to status epilepticus. The only approved out-of-hospital treatment for seizure emergencies is rectal diazepam, but many patients and caregivers object to this route. This project proposes to develop water-based intranasal benzodiazepines that are easily administered and safely deliver high drug concentrations that rapidly terminate seizures.
Read More...

James Cloyd, PharmD has a doctorate in pharmacy and is a Professor and the Lawrence C. Weaver Endowed Chair in the College of Pharmacy at the University of Minnesota. He also is the Director of the Center for Orphan Drug Research in the College of Pharmacy. He has devoted almost 4 decades to research leading to improved treatment of epilepsy including the development of rectal diazepam (Diastat) and intranasal benzodiazepines for rescue therapy.
-
Project Title: Dry Sensor-based Neonatal EEG Monitoring "NEMO"
Tammy Tsuchida, MD, PhD
Children's National Medical Center
Washington, D.C.
Summary: The principal goal of this project is to design, build and test an initial Neonatal EEG Monitor (NEMO) prototype headset that can be subsequently developed into a product ready for FDA 510(k) premarket approval as the first medical-grade neonatal dry sensor system. This easy-to-use headset will fit a range of head shapes, enable EEG recording within 10 minutes, not injure delicate neonatal skin and can be used with any conventional EEG recording systems, thereby increasing availability of monitoring.
Read More...

Tammy N Tsuchida is currently a Clinical Neurophysiologist and Neonatal Neurocritical Care Neurologist at Children's National Health System in Washington, DC. She received her MD, PhD from Columbia College of Physicians and Surgeons' MD, PhD training program and her Child Neurology and Clinical Neurophysiology training from the University of California San Francisco. Further valuable experience was gained at Children's National Health System's Comprehensive Pediatric Epilepsy Program, Neonatal Neurocritical Care Program, and the American Clinical Neurophysiology Society's ICU Monitoring SIG. Her primary interest is management of seizures and epilepsy in neonates and infants. Her goals are to optimize neonatal neuromonitoring and neuroprotective therapies to ultimately improve developmental outcomes and decrease incidence of epilepsy.
-
Project Title: Focused Ultrasound for Subcortical Epilepsy (FUSE) Study
Nathan B. Fountain, MD
University of Virginia
Charlottesville, Virginia
Summary: Transcranial MRI guided focused ultrasound is an emerging technology designed to focus sound waves at a single point in space, resulting in a discrete thermal lesion. This clinical trial will use sound waves (Focused Ultrasound) to selectively destroy a small area of abnormal brain cells deep in the brain, such as hypothalamic hamartomas, that cause seizures. The sound waves are focused by a new technology and directed to the correct location by live ongoing magnetic resonance imaging.
Read More...

Nathan B. Fountain, M.D. is Professor of Neurology and since 1998 has been Director of the F.E. Dreifuss Comprehensive Epilepsy Program at the University of Virginia School of Medicine where he completed neurology residency as well as clinical and research epilepsy fellowships. Dr. Fountain's interventional research interests include experimental therapeutics of unconventional drugs and devices for epilepsy. Other research interests include the natural history, outcome, and pathophysiology of nonconvulsive status epilepticus and studies of the natural history of changes in seizure frequency. He serves on the board of the National Association of Epilepsy Centers and the Epilepsy Foundation Professional Advisory Board.
Spring 2014: Epilepsy Innovation Seal of Excellence
-
Project Title: Epalex Inhaler
Michael Rogawski, MD, PhD
Epalex Corporation
South San Francisco, CA
Summary: Epalex Corporation is developing a pocket-sized inhaler to be used by a person with epilepsy to prevent the occurrence of a potentially serious seizure. When a seizure warning sign (aura) occurs, the inhaler is used to self-administer a powerful antiseizure drug into the lungs, which is rapidly absorbed into the blood stream and carried to the brain, stopping the seizure.
--------
Read More...

Michael A. Rogawski is professor and chair emeritus of the Department of Neurology and a member of the Center for Neuroscience at the University of California, Davis. Until 2006, he was senior investigator and chief of the Epilepsy Research Section at the National Institute of Neurological Disorders and Stroke. Dr. Rogawski received his B.A. from Amherst College, and M.D. and Ph.D. (pharmacology) from Yale University. After serving as a postdoctoral fellow in the Laboratory of Neurophysiology, NINDS, he completed residency training in neurology at Johns Hopkins. Dr. Rogawski's research encompasses cellular neurophysiological studies of ion channels with a focus on the mechanisms of action of antiepileptic drugs); animal models of epilepsy, migraine, and nerve agent intoxication; and clinical studies on new treatments for seizures, epilepsy, migraine, traumatic brain injury, and neurodevelopmental disorders. His laboratory studies on AMPA receptors and neurosteroids have led to new treatment approaches for seizures and epilepsy.
Dr. Rogawski has received the NIH Director's Award, the Epilepsy Research Award for Outstanding Contributions to the Pharmacology of Antiepileptic Drugs from the American Society for Pharmacology and Experimental Therapeutics, and the Service Award from the American Epilepsy Society. He presented the British Pharmacological Society Lecture, the Killam Lecture of the Montreal Neurological Institute, and the American Epilepsy Society's William G. Lennox Lecture. Dr. Rogawski has served on the editorial boards of Molecular Pharmacology, Epilepsia, Epilepsy Research, Synapse, CNS Drug Reviews, Current Neuropharmacology, Pharmacology & Therapeutics, BMC Pharmacology, Cellular and Molecular Neurobiology, and Nature Scientific Reports, and was associate editor of Neurotherapeutics. He is a founder and was co-chief editor of Epilepsy Currents, the journal of the American Epilepsy Society. He has served on the board of directors of the American Epilepsy Society, he has been a member of advisory panels to the National Institutes of Health, and he serves in an advisory capacity as a special government employee to the Food and Drug Administration. He is past president of the American Society for Experimental NeuroTherapeutics.
June 2014: Shark Tank Competition
- Project Title: An In-home, Inexpensive Anti-epileptic Drug-monitoring Device using Saliva
Oren Knopfmacher, PhD
Avails Medical, Inc.
Palo Alto, CA
Summary: Therapeutic monitoring of anti-seizure medication is crucial to determine optimum drug dosage in epileptic patients and to avoid seizures and periods of toxicity. Current drug monitoring is invasive it requires a clinic visit and is utilized only infrequently by patients. Avails Medical overcomes this hurdle by providing a novel device, allowing simple, non-invasive and convenient in-home drug monitoring using saliva as the target body fluid. This project empowers patients to keep their drug dose optimal at home by noninvasively and immediately assaying levels of anti-seizure medication in saliva.
Read More...

Oren Knopfmacher obtained his PhD in experimental physics from the University of Basel in Switzerland. In his dissertation, he focused on novel types of bio-chemical (nano-) sensors. Subsequently, he moved to California where he worked for the past two 2.5 years as a postdoc on new types of flexible biosensors at Stanford University in the group of Prof. Zhenan Bao. After attending the Ignite program of the Stanford business school, he founded Avails Medical, Inc. to make his sensor technology available to the larger community to improve their quality of life.

Dr. Fisher is Maslah Saul MD Professor and Director of the Stanford Epilepsy Center. He had research awards from the Klingenstein Foundation, EF, CURE and NIH. He published 160 peer-reviewed articles and 5 books. He was named 1996-2013 in Best Doctors in America. He received the Ambassador Award from the International League Against Epilepsy, the 2005 AES Service Award and the 2006 Annual Clinical Research Award. Dr. Fisher is Past-President of the American Epilepsy Society, and has served on the Board of the ILAE and as Editor-in-Chief of the Journal, Epilepsia. He is immediate past Editor-in-Chief of the website epilepsy.com. His research is on new devices and drugs to treat epilepsy.
- Project Title: Shower Power
Jon Davis
Kansas City, Missouri
Summary: Shower Power, intends to provide safety and peace-of-mind while showering. Primarily for people with epilepsy, this device could also be useful for aging and adolescent groups. The Shower Power system checks for normal upright body position within the shower and will sound a variety of alerts to warn caregivers that the body's position is no longer upright. Since Shower Power non-invasively monitors body position within the shower, no alterations to one's daily shower routine is required.
Read More...

Jon Davis was born in Spokane, Washington. Early on, he was intrigued with how things worked which has carried on throughout his life. He enjoyed participating in Boy Scouts and he earned their highest award, Eagle Scout. After high school, he moved to Las Vegas, Nevada, where he enrolled in the Chemistry program at the University of Nevada – Las Vegas. He obtained a BS in Chemistry (with biochemistry focus) in 1993. Soon after, he moved to Kansas City and was married. His first R&D chemistry job was with Bayer Cropscience. In 1994, he joined Trinity Biotech and has been in technical management for the last 16+ years. He and his wife still reside in Kansas City with their 2 daughters (Lydia and Claire). Jon is currently enrolled in the MS Chemistry (Analytical) program at the University of Missouri – Kansas City. His wife and children are his daily inspiration.
- Project Title: Seizario - Using Smartphones to Detect Seizures
Shark Tank People's Choice Award
Ahmed and Amir Helmy
University of Florida
Summary: Seizario is a mobile application designed for the purpose of aiding epileptic patients, their families and caregivers in managing their daily lives effectively, using smartphones. Seizario aims to offer two main features; automatic detection of several emergency scenarios, and easy and immediate communication of critical information to family members and caregivers. First, Seizario offers an accelerometer-based learning algorithm coupled with a finite-state--machine to automatically detect grand mal seizures and harmful falls. Warning and alert messages are triggered when potentially dangerous situations are detected. Second, immediate or timed emergency messages can be sent at will to pre--identified family members or caregivers, with (instantaneous or longitudinal) activity, time and location information. Using Seizario regularly should reduce the effect of detected seizures by reducing the response delay due to timely alert notifications. It also provides a tool (bread crumbs) for frequent information notification to loved ones. In addition, it presents long-term detailed logs to caregivers and doctors for continuous behavioral analysis, for improved treatment outcomes.
Read More...

Ahmed Helmy is a Professor of Computer and Information Science and Engineering (CISE) at the University of Florida (UF). He received his doctorate (Ph.D.) degree in Computer Science from the University of Southern California (USC). He was a key researcher in the Network Simulator (NS-2) and Protocol Independent Multicast (PIM) projects at ISIUSC. He founded and directed the Wireless & Sensor Networks Laboratories at USC and UF. He was on the faculty at the Electrical Engineering-Systems Department at USC before joining UF in 2006. He is a recipient of the National Science Foundation (NSF) CAREER Award. He has co-authored over 175 peer-reviewed publications. His research has high impact (over 11,000 citations on Google Scholar), and has achieved wide recognition and earned various awards from the IEEE and ACM professional associations. In June 2014, he and the Seizario team, won the Epilepsy Foundation Shark Tank (innovation) competition, the ACM MobiCom 2014 Mobile App Competition (First place), and Start-up Pitch Competition (Runner- up).

Amir Helmy is a student at the International Baccalaureate (IB) program at Eastside High School in Gainesville, Florida. He has developed several mobile apps, including Seizario and HeartEra. For these apps, he received the Intel science-fair award in 2013 and 2014, the Jr. Hacker-of the-Year awards in 2013 and 2014, first place in school and regional science fairs in 2013 and 2014, and first place in computer science at the Florida state science fair in 2013. He was also a semi-finalist for the Broadcom Masters competition in 2013 and 2014, and a semi-finalist at the ACM MobiCom conference app competition in 2013.
In 2014, he was the first place winner of the ACM MobiCom Mobile App Competition, and the runner-up (2nd place) winner of the Start-up Pitch Competition. In June 2014, as a key part of the Seizario team, Amir won the Epilepsy Foundation Shark Tank (innovation) competition.
In addition, he has other interests in chess, robotics and jazz (tenor saxophone). He led his robotics team to win the FLL (First Lego League) robotic competition design regional award in 2012, was part of the school team winning 2nd place in the national chess championship, and was on all state jazz-band for Florida in 2014. He also co-created Seizario.com.
December 2013: Epilepsy Innovation Seal of Excellence
- Project Title: Advancing Extended NeuroAmides to Clinical Trials
Harold Kohn, PhD
NeuroGate Therapeutics, Inc.
Raleigh, NC
Summary: Epilepsy is a neurological disorder for which current medications are ineffective for many patients. We have advanced Extended NeuroAmides (ENAs), a novel compound class that exhibit potent anticonvulsant activities and a unique pharmacological mechanism. Our investigation provides a critical path for ENA development and selection allowing for IND-enabling studies.
Read More...

Harold Kohn is Kenan Distinguished Professor at the University of North Carolina at Chapel Hill where he is professor in the Division of Chemical Biology and Medicinal Chemistry at the Eshelman School of Pharmacy and the Department of Chemistry. His laboratory has focused on elucidating the mode of action of clinical agents (e.g.; anticancer, antibiotic, neurological agents) and drug discovery, and is credited with the discovery of lacosamide (Vimpat®), a first-in-class antiepileptic drug marketed world-wide for the treatment of partial-onset seizures in adults. Studies by Dr. Kohn and his coworkers have led to more than 175 peer-reviewed studies published in leading journals, 9 US patents and more than 25 abroad. Kohn received a bachelor of science in chemistry degree from the University of Michigan in 1966 and a PhD degree in chemistry from the Pennsylvania State University in 1971. Dr. Kohn's studies have provided the framework for the training of 30 doctoral, 7 master, and 30 postdoctoral students. Kohn's research contributions have been recognized with the Alfred P. Sloan Research Fellowship, the Camille Dreyfus Teacher Scholar Award, and the American Association of Colleges of Pharmacy Paul R. Dawson Biotechnology Award. In 2011, Kohn founded NeuroGate Therapeutics, Inc. to foster the development of new low-molecular compounds to treat disorders affected by neuronal hyperexcitability.
- Project Title: Novel Long Term Effective (LT) GABA PAMs for Refractory Epilepsy
Tom Kronbach, PhD
BioCrea GmbH
Radebeul, Germany
Summary: BioCrea's LT GABA PAMs offer a new way to treat refractory epilepsy: They modulate the same validated target as benzodiazepines, but in a different way, leading to reduced side effects combined with maintained efficacy. This will enable effective and long term treatment of patients with unmet need.
Read More...

Tom founded BioCrea in 2010 as a management-buy-out from Biotie Therapies. He established an international management team to produce first-in-class drugs from leading scientific concepts with robust and proven lead optimization skills. Previously, Tom was a founder and CSO of elbion Group, which was acquired by Biotie Therapies in 2008, where he continued as CSO and Managing Director. Tom played the key role in the conception and negotiation of deals and collaborations with Boehringer Ingelheim, Pfizer, Wyeth and GlaxoSmithKline.
December 2013: New Therapy Grant Awards
- Project Title: Counting Seizures in Outpatients using a Wrist-worn Multimodal Sensor
Daniel Friedman, MD
New York University School of Medicine
New York, NY, United States
Summary: This study will exam if a new kind of sensor worn on the wrist can easily and noninvasively detect seizures. A reliable and easy to use seizure-sensor can help physicians, patients and researchers to determine the response to treatment without have to rely on the patient's report, which may be inaccurate.
Read More...

Dr. Friedman is an Assistant Professor of Neurology and an adult epileptologist at the NYU Langone Comprehensive Epilepsy Center. His clinical interests include the surgical treatment of epilepsy. He also has active research interests in understanding epilepsy-related mortality, including prevention strategies, and methods to improve trials of epilepsy therapy.
July 2013: Epilepsy Innovation Seal of Excellence
- Project Title: Airbag Head Protection for People with Epilepsy
Hovding Sverige AB
Malmoe, Sweden
Klas Josephson, PhD
Summary: This project starts from the airbag for cyclists, Hovding, that protects the cyclist's head in case of an accident. The aim is to take this technology and adapt it for people with epilepsy. This consists of two steps, to adjust the accident detection system to fit people of epilepsy and to develop new airbag that is rechargeable and reusable. The second step will make the product more affordable for the user.
- Project Title: New Anticonvulsant with a Broad Spectrum of Activity
AurimMed Pharma, Inc.
Salt Lake City, UT
Amir Pesyan, M.Sc.H.
Summary: In animal studies done by the National Institutes of Health (NIH), the new antiepileptic drug, AMP-X-0079, has shown activity against a larger number of different seizure types than any drug currently on the market. This project will complete the studies required by the FDA so that human testing can begin.
June 2013: New Therapy Grant Awards
- Project Title: Factors Setermining Placebo response in drug-resistant focal epilepsy
Emilia Bagiella, PhD
Mount Sinai School of Medicine
New York, NY
Summary: The magnitude of placebo response is an important factor in the outcome of clinical trials, and an inflated placebo response can obscure true drug-placebo differences. Failure to demonstrate drug–placebo differences where true differences exist encourages sponsors to terminate drug development programs prematurely, thus preventing patient access to effective treatments. In this project we propose to analyze a large sample of data to determine the factors that affect placebo response in clinical trials.
- Project Title: MR-guided focused ultrasound for treatment of mesial TLE
Ryder Gwinn, MD
Swedish Neuroscience Institute
Seattle, WA
Summary: This project aims to be the world's first clinical investigation of MR-guided focused ultrasound as a potential completely noninvasive and radiation-free treatment alternative for medication refractory patients with mesial temporal lobe epilepsy. The procedure would require no scalp incision, burr holes, electrodes, or ionizing radiation, and thus limit the risk of complications as compared to other surgical, minimally-invasive (e.g. Visualase) or radiosurgery (e.g. Gamma Knife) procedures. Positive clinical results have been shown by a recently completed pilot study of focused ultrasound for the treatment of essential tremor and other pilot trials for functional targets are currently under way.
- Project Title: Two open-label studies of CBD in Dravet and Lennox-Gastaut syndromes
Catherine Jacobson, PhD
Stanford University
Stanford, CA
Summary: This is a joint project between four pediatric epilepsy experts (Dr. Cilio - UCSF, Dr. Devinsky - NYU, Dr. Thiele - Massachusetts General, and Dr. Cross - Great Ormond Street Hospital) and GW Pharma to investigate the safety and tolerability of a novel anti-epileptic drug, Cannabidiol (CBD). Further, the two studies outlined in this proposal will provide a first look at efficacy in seizure control in two severe childhood epilepsy syndromes – Dravet and Lennox-Gastaut.
- Project Title: Minimally invasive mapping and ablation to treat epilepsy
Gregory Worrell, MD, PhD
Mayo Clinic
Rochester, NY
Summary: Minimally invasive surgical techniques have revolutionized many areas of medicine; however, open surgery for epilepsy has remained unchanged for decades. We propose minimally invasive catheter-based methods to replicate excellent outcomes of epilepsy surgery. Our goals are improved outcomes, lower morbidity, lower cost and greater access to epilepsy diagnosis and treatment.
December 2012: New Therapy Grant Award
- Project Title: HE3286 Treatment of Drug Resistant Epilepsy
Clarence Ahlem, M.S., Vice President, and team
Harbor Therapeutics, Inc.
San Diego, CA
Summary: This grant supports the preclinical development of Harbor Therapeutic's anti-inflammatory drug HE3286 in drug resistant epilepsy. HE3286 is currently in Phase II clinical trials for diabetes. Brain inflammation is linked to epilepsy including drug resistant seizures, and specific anti-inflammatory drugs have been able to suppress ongoing seizures in animal models of chronic epilepsy. HE3286 is an orally bioavailable, blood-brain-barrier permeable anti-inflammatory compound that is being repurposed for epilepsy. The anti-inflammatory action of HE3286 demonstrated in several different inflammatory disease models—including Parkinson's disease and multiple sclerosis—provides a strong rationale for its evaluation in epilepsy.
The specific study to be performed will be a preclinical study in chronic seizures in mice—specifically in a model that captures the complex chronic inflammatory interactions which have been shown to be targeted by the mechanism of action of this compound in other disease models. The study will be performed by Annamaria Vezzani, PhD, Head of the Laboratory of Experimental Neurology at the world-renowned Mario Negri Institute for Pharmacological Research, Milan, Italy. Dr. Vezzani maintains one of the few laboratories in the world with significant experience in the mouse model of chronic epilepsy. The proposed experiments in mice will be completed within one year of the grant award.
June 2012: New Therapy Grant Awards
- Project Title: High Definition Cathodal Transcranial Direct Current for Treatment of Focal Status Epilepticus
Alexander Rotenberg, M.D., Ph.D., Assistant Professor of Neurology, Children's Hospital Boston, and team
Soterix Medical Inc.
New York, NY
Summary: The team is developing a novel, non-invasive form of cathodal transcranial direct current (tDCS), a painless and safe method for focal brain stimulation. Despite its favorable safety profile and a proven capacity to modulate cortical excitability, conventional tDCS technology has not successfully been applied to the treatment of focal status epilepticus (FSE), in part due to the poor spatial targeting. The research team proposes to overcome this limitation with High Definition (HD)-tDCS and hypothesizes that HD-tDCS can safely reduce seizure burden in patients with FSE. FSE is characterized b y repetitive discrete seizures often lasting more than an hour (at times as long as days or weeks). Unfortunately, drug treatments are often ineffective.
HD-tDCS devices are lightweight, inexpensive and can be applied in minutes with minimal training. The proposed HD-tDCS platform is the only neuromodulation technology capable of highly focused DC stimulation of identified cortical targets, an essential safety feature for populations such as children with epilepsy. Favorable data from the proposed studies may enable larger trials of cathodal HD-tDCS in emergency seizure settings. Of note, clinical applications for additional use of this technology in the management of mood disorder and chronic pain are also in progress.
- Project Title: Direct Network Visualization of Drug Efficacy Using of MRI
Jin Hyung Lee, Ph.D., Assistant Professor, Neurology and Neurological Sciences, Bioengineering, and team
Stanford University
Stanford, CA
Summary: The research team has utilized a revolutionary technology, optogenic functional MRI (ofMRI), to directly visualize--in vivo--the actual spread of seizure activity through brain networks as well as drug effects on this seizure activity. The approach is to create an optogenic seizure rat model by implanting a fiber-optic light source that can create seizures on demand. The ofMRI imaging techniques would then display the specific brain networks activated by the seizures, enabling precise knowledge about which areas of the brain are affected. The technology may be used to expedite preliminary evaluation of drugs and their potential value in neurological disease. More specifically, with the potential to better visualize brain region involvement and response, ofMRI may expedite drug screening and reduce clinical trial costs by allowing better drug selection. The funding will be applied to testing several drugs in two proprietary animal models with the objective of accelerating the development of new neuroactive drugs and treatments for epilepsy.
- Project Title: Validation of the ANI-SI Dry EEG Headset in Time-Critical Applications
Wendy Catharina Ziai, M.D., M.P.H., Assistant Professor, Neurology, Anesthesiology and Critical Care Medicine, Johns Hopkins University School of Medicine and team with participating institution University of California, San Francisco
Advanced Neurometrics, Inc.
San Diego, CA
Summary: The team will test Advanced Neurometrics' ANI-SI EEG system in ER and intensive care units to evaluate how the device performs in the measurement and characterization of seizures and other causes of altered mental states compared to standard EEG. The ANI-SI EEG system is a dry electrode headset that records EEG without requiring extensive and time-consuming patient skin preparation, head measurements or gels. Timely identification of the presence or absence of ongoing electrographic seizures is critical for appropriate clinical care; delays in diagnosis may result in a worse neurologic outcome. Most emergency rooms and hospitals lack the capability for emergency EEG, which may be the only way to confirm if a patient is experiencing a seizure or if decreased alertness is due to another condition. The ANI -SI EEG system has thus far proven to record EEG signals with high fidelity and has demonstrated significant promise in reducing time to interpretable EEG data compared to wet electrode conventional EEG.
- Project Title: Wireless EEG Seizure Patch
Mark Lehmkuhle, Ph.D., Chief Executive Officer, Chief Technology Officer, and team
Epitel, Inc.
Salt Lake City, UT
Summary: The team is conducting an expanded Phase 1 clinical study of a novel EEG device with potential to track the frequency of drug-refractory epileptic seizures. Designed as an affordable wireless patch, the device is small enough to be placed on the scalp for the identification of abnormal brain activity in neonates, children and adults without requiring the use of protective head gear or electrode wires. The low-power transmitter signal is received by a USB sensor attached to a conventional smart phone, which is used to verify signal quality. The device can log EEG activity for approximately 12 days permitting users to proceed with their daily routines. In contrast to current ambulatory EEG technology, this patch technology is small and discrete, easy to conceal, and eliminates the social stigma associated with ambulatory and outpatient monitoring.
The Epitel wireless EEG transmitter with data logger is the first device designed to yield long-term data on seizure frequency for patients whose seizures involve both he mispheres, such as patients with complex partial seizures. The grant will support feasibility studies for tracking seizures in patients with refractory epilepsy based on this relatively low-cost, unobtrusive and patient-sensitive advanced technology. With improved and high-quality access to quantitative data of seizure frequency and pattern and seizure history, the Epitel wireless device has the promise of better treatment outcomes and may serve as a valuable screening tool for clinical trials.
November 2011: New Therapy Grant Awards
- Project Title: Phase IIA Clinical Trial for Add-on Triheptanoin
Karin Borges, PhD, and laboratory team
The University of Queensland
St. Lucia, QLD, Australia
Summary: The team recently found that a synthetic edible tasteless oil inhibits seizures in mice. This grant will allow her in collaboration with Prof. Dr. Terence O'Brien, and other epileptology colleagues from Melbourne, Australia, to investigate if this oil can reduce seizures in adult patients with medically refractory epilepsy. This clinical trial will take place in Melbourne, Australia.
- Project Title: Novel Inferior Alveolar Nerve Stimulation Device
Ming Cheng, MD
NuroRestore
Brown University Medical School
Mansfield, MA
Summary: The researcher has developed a new epilepsy device that works like a pacemaker, but stimulates a nerve in the lower jaw instead of the heart. This stimulation seems to decrease seizures in animals and humans. We implanted this device in a 41 year old woman with epilepsy, and it completely changed her life. We now plan to implant more patients in clinical trials, with the hope that this will lead to a new option for epilepsy sufferers worldwide.
- Project Title: XeriJect Diazepam for Emergency Treatment of Seizures
Steven Prestrelski, PhD
Xeris Pharmaceuticals, Inc
Austin, TX
Summary: The goal of this project is to develop a low volume, painless, auto-injectable formulation for the subcutaneous administration of diazepam to treat seizures in epileptics, similar to the EpiPen™ for treatment of severe allergic reactions. Development of a simple, ready-to-use auto-injectable diazepam would yield an emergency medication with significant advantages over the current standard of care.
- Project Title: FDA Application for Epilert V1
Amos Shaham, MS
BioLert Ltd.
Even Yehuda, Israel
Summary: This EpiLert project takes a unique approach to the detection of epileptic seizures. A system that includes a small electronic movement sensor unit worn by the patient and a compact alert unit carried by a caregiver is hereby suggested. The set is called EpiLert, and includes a 3 axis accelerometer chip that transfers the seizure's movements to electrical signals and a microprocessor that utilizes a mathematical algorithm to identify seizures. The algorithm (patent pending) that can differentiate an epileptic movement from all other movements is the key issue to the reliability and successful adoption by families and patients of such a system.
April 2011: New Therapy Grant Awards
- Project Title: 1 Hz rTMS for Treatment of Temporal Lobe Epilepsy
Alexander Rotenberg, MD, PhD, Assistant Professor of Neurology
Children's Hospital Boston
Boston, MA
Summary: The researcher received a grant to advance a new non-invasive magnetic coil that delivers deep brain stimulation as a new approach to treating refractory epilepsy. The grant will support a clinical study to evaluate the novel repetitive transcranial magnetic stimulation (rTMS) H-Coil as a promising non-invasive method of inhibiting the abnormal electrical and neuotransmitter activity believed to underlie seizures in focal temporal lobe epilepsy (TLE). A majority of the patients with seizures originating in this part of the brain are difficult to treat and/or resistant to existing therapies.
November 2010: New Therapy Grant Awards
- Project Title: SmartWatch: Seizure Detection
Chandan Gope, PhD
Smart Monitor Corporation
San Jose, CA
Summary: The awardee received a grant for the SmartWatch, a novel device that continuously monitors, detects, alerts upon and records rhythmic, repetitive convulsive movements of the limbs, caused by a generalized tonic-clonic or grand-mal seizure. It is low cost, passive, non-invasive device that is easy to use. It contains a miniaturized 3D motion / accelerometer sensor that detects fine and gross movements of the body part (arm wrist or ankle) on which the watch is worn. A mathematical detection algorithm embedded in the Smart Watch analyzes the movements to determine if they are consistent with those caused by a seizure. This grant will continue patient testing and commercialization for the SmartWatch.
- Project Title: EpiLert Seizure Alert Device
Amos Shaham, MS
Biolert Ltd.
Even Yehuda, Israel
Summary: The awardee received a grant to refine algorithms for the Epilepsy Alert device based on limb movement, for which a robust seizure identification algorithm is an essential element. BioLert has developed and refined these algorithms from a database of seizure and non-seizure movements that were accumulated via unique hardware and software in a hospital setting. In this proposed project the algorithm is to be embedded in the microprocessor that is incorporated in the EpiLert set. As part of this project, prototypes of the EpiLert sets will also be constructed which will include a Sensor Unit with a form factor similar to a wristwatch, and an Alert Unit the size of a cell-phone. These prototypes will be further evaluated in hospitals to confirm performance.
- Project Title: MRI-guided Laser Ablation of Epilepsy Foci
Ashok Gowda, PhD
Visualase, Inc.
Houston, TX
Summary: The awardee received a grant to study the feasibility of MRI-guided laser ablation of epileptogenic seizure foci as a treatment for patients refractory to pharmacologic therapies. For many of these patients (who constitute approximately one third of all epileptic patients), surgical resection offers a potentially curative therapy, yet few patients elect to undergo resection due to the invasive nature of the procedure and risk for morbidity. The hypothesis is that precise destruction of epileptogenic foci using a minimally invasive technique known as laser induced thermal therapy (LITT) would provide results approaching surgical resection in terms of seizure relief, and could be carried out with a far lower risk of surgical morbidity to the patient. The possibility of a minimally invasive ablation procedure would provide medically intractable patients with an alternative to more invasive surgical procedures while keeping open all possible therapy options in the future including subsequent ablation procedures if necessary.
May 2010: New Therapy Grant Awards
- Project Title: Galanin Gene Delivery to the Hippocampus for Mesial Temporal Lobe Epilepsy
Scott McPhee, PhD
Asklepios BioPharmaceutical, Inc.
Chapel Hill, NC
Summary: The awardee received a grant to test a novel gene therapy to directly target epileptogenic hippocampal tissue. This approach could be a therapeutic for epilepsy patients whose options are limited to a brain resection. The proposed study will test adeno-associated virus (AAV) based galanin gene delivery. Brain distribution studies will validate a dose extrapolation strategy for preclinical and clinical development, definitive preclinical studies evaluating toxicology and biodistribution. These will lead to the FDA submission process before clinical testing may be initiated.
- Project Title: Intracranial EEG Acquisition System with Online Fast Ripple Detection
Catherine Schevon, MD, Assistant Professor, Department of Neurology
Columbia University Medical Center
New York, NY
Summary: The researcher received a grant to continue to study high frequency oscillations in the brain which can identify areas for epilepsy surgery treatments, but are technically difficult to detect. The system can bring automatic online HFO detection into clinical practice, making current surgical treatments more effective, and potentially simplifying surgeries for many epilepsy syndromes.
November 2009: New Therapy Grant Award
- Grant - Supplemental Request: Development of IV Topiramate for Neonatal Seizures
James C. Cloyd, PharmD, Professor and Director, Center for Orphan Drugs
University of Minnesota - Twin Cities
Minneapolis, MN
Summary: The awardee received a grant to continue to pursue the development of an intravenous (IV) formulation of topiramate to bring a well-established and effective medication to a new therapeutic indication: neonatal seizures. Neonatal seizures, for which there are limited therapeutic options, can result in impairment in development, cognition and potentially harmful side effects to the developing brain. Laboratory studies offer compelling evidence that topiramate, an effective anti-epileptic drug with a good safety record in children over two years of age and adults, could substantially improve the management of seizures in newborn infants. An IV formulation would be required to treat neonates because of the need to precisely control drug concentrations, and no IV topiramate currently exists.
November 2009: New Therapy and Milken Family Foundation Grant Award
- Project Title: Proof of Principle Trial: 2-deoxy-D-glucose for Intractable Seizures
Nathan Fountain, MD, Professor and Director, F.E. Dreyfuss Comprehensive Epilepsy Program
University of Virginia
Charlottesville, VA
Summary: The awardee received a grant to conduct a preliminary human clinical trial to determine if 2DG, an analogue of normal sugar that blocks sugar metabolism, reduces seizures in people with frequent seizures. Despite 11 new drugs for epilepsy since 1990, ~50% of patients have recurring seizures and ~15% remain intractable. 2DG has novel acute anticonvulsant and chronic antiepileptic actions, including enhanced delivery into brain regions undergoing seizures enabling effective "post-seizure" treatment. 2DG is distinctive compared to ALL currently marketed anticonvulsants, and appears to have potential to increase the number of patients who achieve control, and to favorably modify adverse consequences in patients in whom complete control is not achieved.
November 2009: Epilepsy Therapy Project - Investment
- NeuroTherapeutics Pharma
Chicago, Illinois
NeuroTherapeutics Pharma is a privately held life sciences company focused on the discovery and development of novel therapeutics for patients suffering from CNS disorders.
Summary: NTP will file an IND for its lead compound, NTP-2014, in the first quarter of 2010. The compound has exhibited excellent efficacy results in multiple models of epilepsy, chronic neuropathic pain and acute pain. NTP-2014 also has demonstrated a very favorable safety profile in the IND-enabling (GLP) subchronic toxicology studies. NTP is financially backed by Novo Ventures and Thomas, McNerney and Partners. The company is led by a strong management team with significant drug development experience in the CNS space. Stephen Collins, MD, PhD is President and Chief Executive Officer.
July 2009: New Therapy Grant Awards
- Project Title: Safety Profile of the Subdural Hybrid Neuroprosthesis for Focal Epilepsy
Ludvig Nandor, MD, PhD, Associate Professor
New York University School of Medicine
New York, NY
Summary: The awardee received a grant to determine the safety of a new medical device, the Subdural Hybrid Neuroprosthesis, for the treatment of drug resistant, surgically untreatable focal epilepsies. The device will deliver anti-seizure drugs directly into the seizure focus to prevent seizures without side-effects. The safety of this emerging epilepsy therapy will be tested in monkeys so that the device can be implanted in the same way in humans.
- Project Title: Clinical EEG Acquisition Systems with Online Fast Ripple Detection
Ludvig Nandor, MD, PhD, Associate Professor
Columbia University Medical Center
New York, NY
Summary: The awardee received a grant to develop a practical intracranial EEG recording system that will bring information on high frequency EEG signals into clinical practice, thus increasing the efficacy of the current surgical treatment of medically refractory partial epilepsy. By more specifically identifying the epileptogenic region, seizure outcomes can be improved while minimizing the area of brain that must be removed surgically. If these frequencies are found to be a reliable biomarker in interictal recordings, this could obviate the need for prolonged implantation, or even single-stage surgical procedures for neocortical epilepsy syndromes.
December 2008: New Therapy Grant Awards
- Project Title: Smart Watch - Seizure Monitor
Chandan Gope, PhD
IntelliVision Technologies, Corp
San Jose, CA
Summary: The awardee received a grant to define the usefulness of the "Smart Watch" - a passive, non-intrusive seizure detection device for home or hospital use. The Smart Watch provides early detection & alerting to care-givers of epileptic patients prone to tonic-clonic or tonic seizures, enabling early intervention that can protect the seizing person. The study will validate that seizures are detected, and provide information for people interested in using the system when it is commercially available.
- Project Title: Novel Tellurium Compounds with Neuroprotective Activity in Epilepsy
Benjamin Sredni, PhD
Bar Ilan University
Ramat Gan, Israel
Summary: The awardee will use this grant to assess anti-seizure effectiveness of AS101 and SAS, two novel tellurium-based drugs with multifunctional traits, targeting both inflammatory and death of brain cells. These drug characteristics, coupled with their excellent safety profile in clinical trials in other disorders, suggest the tellurium compounds as promising agents for the treatment of epilepsy. The study will evaluate efficacy of the tellurium compounds in dogs with epilepsy.
December 2008: New Therapy and Milken Family Foundation Grant Award
- Project Title: Assessment of Narrow Spectrum Antiepileptic Drugs in the Photosensitivity Model
Jacqueline French, MD
New York University
New York, NY
Summary: The awardee received a platform grant, designed to evaluate the usefulness of a human model to determine the efficacy of new medications to stop seizures. The study will determine whether patients who usually have epileptic discharges when exposed to flashing lights (photosensitive patients) will show a reduction in epileptiform brain wave activity when they take a single dose of three antiepileptic drugs known to be effective, but using different mechanisms to affect the brain (carbamazepine, pregabalin, levetiracetam). If all of the known drugs cause a similar pattern, we will know that this type of study may be able to identify promising new drugs for epilepsy.
June 2008: New Therapy and Finding a Cure for Epilepsy and Seizures (FACES) Grant Award
- Project Title: Preclinical New Chemical Entities as Novel Anti-epileptic Drugs Targeting Dynamin
Philip James Robinson, PhD, Unit Head of the Cell Signaling Unit
Children's Medical Research Institute, Sydney, Australia
Adam McClusky, PhD, Associate Professor of Chemistry
University of Newcastle, Australia
Terrence J. O'Brien, MD, Associate Professor of Medicine
University of Melbourne, Melbourne, Australia
Summary: The researchers were awarded the FACES grant. The study is focused on development and commercialization of small molecule inhibitors of dynamin, an enzyme that is a key component of the synaptic vesicle cycle in neurons and is required for sustained neuronal activity. Blocking dynamin is a new approach to treatment of epilepsy because the drugs may only act after a few seconds of prolonged or high brain activity, such as occurring during the initiation or propagation of a seizure. Thus the approach will likely have less effect on the brain when there is no seizure activity. The potential benefits of this may be fewer side effects of dynamin inhibitor-based AEDs and the potential to have an impact on people who are refractory to all other AED treatments.
June 2008: New Therapy Grant Award
- Project Title: Pulmonary Delivery of Propofol for the Control of Intractable Seizures
Michael A. Rogawski, MD, PhD, Professor and Chairman, Department of Neurology
University of California, Davis
Randall Betrand Murphy, Senior Vice President
Medkura Pharmaceuticals
Kirk Nylen, post doctoral fellow
University of California, Davis
Summary: The awardees were the recipients of funding for the development of an inhaler device, like that used in asthma treatment, to abort an impending seizure in a person with epilepsy who experiences seizure warning signs. The inhaler device uses a new formulation of propofol, a powerful anticonvulsant, for delivery through the patient's lungs.
June 2008: New Therapy Grant Award
- Project Title: Convection Enhanced Delivery of Anticonvulsant Toxins for the Treatment of Intractable Partial Epilepsy
Michael A. Rogawski, MD, PhD, Professor and Chairman, Department of Neurology
Dorota Zolkowska, MD, PhD, Project Scientist at the Epilepsy Research Laboratory
University of California, Davis
Davis, CA
Summary: The awardees received a grant for their proposal to deliver anticonvulsant peptides via an implanted catheter using convection enhanced delivery. This is designed to treat focal epilepsy in patients whose seizures are resistant to antiepileptic medications. Monitored by MRI scanning, the infusion provides protection against seizures for three months and can be repeated as necessary.
December 2007: New Therapy Grant Award
- Project Title: Randomized, Double Blind Parallel Trial of External Trigeminal Nerve Stimulation for Intractable Epilepsy
Christopher DeGiorgio, M.D.
Jason Soss, M.D.
Lara Schrader, M.D.
Department of Neurology Seizure Disorders Center
Olive View
UCLA School of Medicine
Los Angeles, California
Summary: The awardees, in collaboration with Todd Whitehurst, M.D., of Advanced Bionics, Inc., were the recipients of a grant to perform a randomized trial of Trigeminal Nerve Stimulation (TNS) in people with poorly controlled epilepsy. Christianne Heck, M.D., of the USC-Keck School of medicine, also will be a co-investigator in this study. TNS is a promising new therapy that involves the external electrical stimulation of a nerve located above the eyes and over the forehead. Neurostimulation is a promising alternative for patients who have failed medical therapy and who are not surgical candidates. Stimulating the trigeminal nerve has several theoretical advantages: it is safe, low cost, and non-invasive. It can easily be performed prior to implantation of a permanent device to determine efficacy. In animals, TNS has a strong antiepileptic effect. In a pilot human study, TNS was safe and well tolerated; in addition, 57% of subjects had a >50% reduction in seizures. This grant will fund a double-blind clinical trial in 50 subjects. It will accelerate development of this exciting and novel therapy, which if successful, could make an innovative therapy available to people with epilepsy worldwide.
December 2007: New Therapy and Milken Family Foundation Grant Award
- Project Title: T-type Calcium Channel Antagonists as Novel AEDS
Jeffrey L. Noebels, M.D., Ph.D., Professor of Neurology, Neuroscience, and Molecular and Human Genetics, Department of Neurology
Baylor College of Medicine
Houston, Texas
Elizabeth Tringham, Ph.D., Project Leader for Epilepsy Discovery Efforts
Neuromed Pharmaceuticals, Ltd.
Vancouver, British Columbia
Summary: The awardees received a grant to pursue the development of selective T-type calcium channel blockers as potential anti-epileptic drugs. Their research is based on evidence that generalized spike-wave absence seizures arise from currents in the brain mediated by the low threshold T-type calcium ion channel, and that these seizures are suppressed when this channel is absent or the current is pharmacologically reduced. This drug discovery project will screen a new class of highly potent and selective T-type calcium ion channel blockers for efficacy in single gene mouse mutant models of human spike-wave absence and temporal lobe epilepsy, with the goal of showing a superior anti-absence profile with fewer side effects than with current therapies for these conditions.
December 2007: Milken Family Foundation Translational Research Award
- Project Title: Pilot Safety Study Evaluating Hippocampal NPY Gene Transfer in Subject with Intractable Mesial Temporal Lobe Epilepsy
Matthew J. During, M.D., Professor and Director, Human Cancer Genetics Program
The Ohio State University
Columbus, Ohio
Neurologix, Inc.
Fort Lee, New Jersey
Summary: The awardees are the recipients of a grant to conduct a pilot study to inhibit seizures caused by temporal lobe epilepsy and to reduce the invasiveness of current surgical treatment approaches by targeting the hippocampus of the brain, and delivering healthy genes where defective ones are causing the central nervous system disorder. In particular, Dr. During and Neurologix will seek to deliver the human Neuropeptide Y (NPY) gene, one of the brain's endogenous anticonvulsants, to neurons in the hippocampus where NPY will act on receptors to inhibit the brain over-activity that occurs during seizures. This approach builds on experience Neurologix has gained through its similar gene transfer approach to treating Parkinson's disease in a Phase 1 clinical trial that demonstrated safety, tolerability and efficacy.
June 2007: New Therapy Grant Awards
- Project Title: Closed-looped Microstimulation with Multi-electrode Arrays to Suppress Epileptic Seizures
Robert E. Gross, M.D., Ph.D., Assistant Professor, Departments of Neurosurgery and Neurology
Emory University School of Medicine
Steve M. Potter, Ph.D., Assistant Professor, Department of Biomedical Engineering, Georgia Institute of Technology and Emory University
Atlanta, Georgia
Summary: The awardees are the recipients of a grant for the development of a novel electrical stimulation approach that directly controls the activity of the brain to attain a more stable state from which seizures will not arise. By continuously controlling the activity of epileptogenic brain areas with distributed low-voltage stimulation, the researchers have shown that small arrays of multiple electrodes can completely suppress epileptic activity in cultured brain tissue. They hope to maintain the brain in a seizure-free or seizure-resistant state, therefore bypassing the need to detect or prevent seizures, and are investigating this treatment in animal models of epilepsy. Funding for this program was made possible in part through a gift from the Patricia Bowman Terwilliger Family Foundation Charitable Trust.
- Project Title: Multi-scale Human Electrophysiology and Stimulation
Gregory A. Worrell, M.D., Ph.D., Associate Professor of Neurology
Mayo Clinic
Rochester, Minnesota
Summary: The awardee received a grant to develop an electrophysiology platform for multi-scale EEG and stimulation experiments. Dr. Worrell and his research team have developed an electroencephalography (EEG) recording and stimulation approach that facilitates investigation across the range of spatiotemporal scales involved in the generation of seizures. They have recently identified a novel electrographic signature of epileptogenic brain that falls outside the spatiotemporal range of conventional intracranial EEG, and that may represent a precursor of seizure generation. They anticipate that their multi-scale EEG and stimulation approach will improve the efficacy of epilepsy surgery and therapeutic brain stimulation.
- Project Title: The Development of Intravenous Topiramate for Neuroprotection and Seizure Control in Neonates
James C. Cloyd, PharmD, Professor and Director, Epilepsy Research & Education Program
University of Minnesota
Minneapolis, Minnesota
Summary: The awardee is the recipient of a grant to pursue the development of an intravenous (IV) formulation of topiramate to bring a well-established and effective medication to a new therapeutic indication: neonatal seizures. Neonatal seizures, for which there are limited therapeutic options, can result in impairment in development, cognition and potentially harmful side effects to the developing brain. Laboratory studies offer compelling evidence that topiramate, an effective anti-epileptic drug with a good safety record in children over two years of age and adults, could substantially improve the management of seizures in newborn infants. An IV formulation would be required to treat neonates because of the need to precisely control drug concentrations, and no IV topiramate currently exists.
December 2006: New Therapy and Milken Family Foundations Grant Awards
- Project Title: Phase IIA Open Label Dose Escalation Study to Investigate the Safety and Tolerability of Huperzine A for the Treatment of Epilepsy
Steven Schachter, MD, Professor of Neurology
Harvard Medical School
Associate Director, Clinical Research
Harvard Medical School Osher Institute
Boston, Massachusetts
Summary: The awardee will pursue a Phase IIA dose-escalation study for the treatment of epilepsy with Huperzine A. This compound, derived from a Chinese herb, is available in the U.S. as a dietary supplement and approved for the treatment of Alzheimer's disease in China, having been administered to over 1,400 healthy volunteers and patients in numerous trials, demonstrating safety and tolerability. Dr. Schachter and colleagues discovered the anticonvulsant properties of Huperzine A in preclinical studies. This study will evaluate this compound as an add-on therapy in patients with refractory epilepsy for its safety and tolerability as well as provide a preliminary assessment of its effectiveness for seizures and its actions on mood and memory.
- Project Title: Galanin-Based Therapy for Refractory Epilepsy
Grzegorz Bulaj, Ph.D., Department of Medicinal Chemistry, College of Pharmacy
University of Utah
South Lake City, Utah
Summary: The awardee received a grant to pursue research in the development of a galanin-based therapy for the treatment of refractory epilepsy. Galanin, a neuropeptide, possesses both anticonvulsant and antiepileptogenic activity when injected directly into the brain. This study aims to chemically synthesize four galanin analogs modified to facilitate transport through the blood brain barrier to overcome this obstacle. These analogs will be evaluated for their biological stability, bioavailability, potential toxicity and anticonvulsant efficacy and potency following intraperitoneal and oral administration. This study is part of a larger preclinical investigation anticipated to result in the selection of one or two Investigational New Drug candidates for advanced preclinical testing.
December 2006: New therapy Grant Award
- Project Title: Adenosine-releasing Brain Implants for Epilepsy Therapy
Detlev Boison, Ph.D., Associate Scientist, Director Epilepsy Program
R.S. Dow Neurobiology Laboratories, Legacy Research
Portland, Oregon
David L. Kaplan, Ph.D., Chair, Department of Biomedical Engineering, Director, Bioengineering & Biotechnology Center
Tufts University
Medford, Massachusetts
Summary: The awardees are the recipients of a grant to pursue the development of adenosine-releasing brain implants for the treatment of temporal lobe epilepsy. Adenosine, an endogenous neuromodulator, has potent anticonvulsive properties and requires long-term delivery directly to the brain. This study aims to develop intraventricular implants of biodegradable silk protein polymers in combination with adenosine for therapeutic delivery of adenosine in a rat model. Both the silk polymer and adenosine are approved by the U.S. Food and Drug Administration for clinical use, and this study aims to engineer a safe delivery system for adenosine that can be employed in clinical feasibility and safety trials.
May 2006: New Therapy Grant Awards
- Project Title: a-methyl-Tryptophan-conjugated Magnetonanoparticles for Enhanced Therapy of Epilepsy
Massoud Akhtari, Ph.D., Semel Institutes of Neuropsychiatry
UCLA School of Medicine
Los Angeles, California
Summary: The awardee received a grant to pursue research with a newly synthesized epilepsy-specific contrast agent for use in improving surgical outcomes. This agent is being developed to selectively tag epileptic brain tissues through MRI imaging. The goal of this project is to provide an opportunity for improved and more accessible surgical therapy for epilepsy through proper localization of epileptic tissues. In addition, this method would enable the monitoring of epilepsies in an effort to identify markers of epileptogenicity and epileptogenesis. Currently no epilepsy-specific contrast agent exists for MRI imaging.
- Project Title: Preclinical, IND-enabling Studies of 2DG for Therapy of Epilepsy
Tom Sutula, M.D., Ph.D., Department of Neurology, University of Wisconsin and Chief Technical Officer
University of Wisconsin and NeuroGenomeX, Inc.
Madison, Wisconsin and Malvern, Pennsylvania
Summary: NeuroGenomeX, Inc., a new neurogenomic sciences company discovering and developing new drug targets for the treatment of disorders associated with neuronal plasticity, has been selected to receive an equity investment, to advance the development of 2-deoxy-D-glucose (2DG), a glucose analog used for decades as an image tracer, as a novel anticonvulsant and disease-modifying treatment for epilepsy. 2DG was recently discovered to have potent acute anticonvulsant and chronic antiepileptic actions including protection against seizure-induced functional alterations in neural circuits. This study is part of a larger preclinical investigation anticipated to result in an Investigational New Drug application before the end of 2007.
December 2005: New Therapy Grant Awards
- Project Title: In Vitro and In Vivo Testing of Herbal Extracts and Extract-derived Compounds for Treatment of Epilepsy
Steven C. Schachter, M.D., Professor of Neurology and Associate Director, Clinical Research
Harvard Medical School Osher Institute
Boston, Massachusetts
Summary: The awardee will identify and obtain anticonvulsant extracts from Asia, Africa and South America and new, potentially novel extract-derived anticonvulsant compounds through an international collaboration with university scientists in Japan, South Korea, Taiwan, Chile, China and Senegal. Herbal extracts and compounds isolated from them with demonstrated efficacy in animal models of epilepsy will be targeted for clinical trials. The project builds on Dr. Schachter's previous experience with East Asian herbal medicines and establishes a systematic global search for novel therapeutic agents for epilepsy derived from herbal sources.
- Project Title: Phase Resetting using DC Stimulation for Seizure Blockage
Ivan Osorio, M.D.
Department of Neurology
University of Kansas Medical Center
Kansas City, Kansas
Summary: The awardee will pursue new research leading to the development of a prototype device that senses the evolving abnormal electrical discharge in the brain associated with seizures and returns it to normal electrical function. Termed "phase resetting" using direct current (DC) stimulation for seizure blockage, this project represents the first use of DC stimulation within the brain as a potential treatment for epilepsy.
- Project Title: Second Generation Antiepileptic Devices: Designing Greater Efficacy
Brian Litt, M.D., Associate Professor of Neurology and Bioengineering
Marc Dichter, M.D., Ph.D., Professor of Neurology
University of Pennsylvania
Philadelphia, Pennsylvania
Summary: The awardees will investigate the translation of existing electrical stimulation therapeutic technology to more effective second-generation devices. The goal of this project is to improve the detection, prediction and stimulation functions of current implantable antiepileptic devices, increasing their efficacy and enabling more patients on these devices to become seizure free.
December 2005: Milken Family Foundation Transitional Research Awards
- Project Title: Targeting Glycolysis as a New Approach to the Treatment of Epilepsy
Xiao-Yuan Lian, Ph.D., Instructor
Janet L. Stringer, M.D., Ph.D., Associate Professor of Pharmacology and Neuroscience
Baylor College of Medicine
Houston, Texas
Summary: The awardees will investigate the manipulation of glucose metabolism as a new treatment for seizures. The project is based on earlier research that suggests the inhibition of glycolysis in brain cells could explain the potent anti-seizure effect of the ketogenic diet. The project is designed to demonstrate the anticonvulsant action of a chemical agent known to inhibit glycolysis and open the door to a new class of therapeutic agents potentially offering improved seizure control with fewer side effects than existing therapies.
- Project Title: Seizure Prevention using BK Channel Antagonists
Alison L. Barth, Ph.D., Assistant Professor, Biological Sciences
Carnegie Mellon University
Member of the Center for the Neural Basis of Cognition, a partnership of Carnegie Mellon and the University of Pittsburgh
Pittsburgh, Pennsylvania
Summary: The recipient was awarded a Milken grant for research on the development of a new therapy with the potential to reverse an acquired channelopathy that increases neuronal excitability and the risk of a chronic seizure disorder following a first seizure.
May 2005: New Therapy Grant Awards
- Project Title: Seizure Control by Closed-Loop Feedback in a Rat Model of Chronic Epilepsy
Leon Iasemidis, Ph.D., Associate Professor of Bioengineering, Harrington Department of Bioengineering
Kostas Tsakalis, Ph.D., Professor of Electrical Engineering
David Treiman, M.D., Newsome Chair in Epileptology, Vice-Chair of the Department of Neurology, Director of the Epilepsy Center at Barrow Neurological Institute, Research Professor of Bioengineering
Arizona State University
Tempe, Arizona
Summary: The researchers were awarded a grant, partially funded through the generous support of the Ali Paris Fund for Landau-Kleffner Syndrome Research & Education, to explore and test a closed-loop electrical stimulation method on an animal model of chronic epilepsy using seizure prediction and intervention algorithms. Dr. Iasemidis', Dr. Tsakalis', and Dr. Treimans' project is based on previous research findings that identified a mathematical model designed to predict seizures in both humans and animals as well as help determine seizure control. This project aims to predict and avert seizures long before their clinical and/or electrographic onset.
- Project Title: Efficacy of Talampanel in Neonatal Seizures and Brain Injury
Frances E. Jensen, M.D., Associate Professor of Neurology
Children's Hospital and Harvard Medical School
Boston, Massachusetts
Michael R. Fetell, M.D., Vice President Oncology and CNS
IVAX Research, Inc.
Miami, Florida
Summary: The researchers were awarded a collaborative grant using an animal model to assess the anticonvulsant and antiepileptogenic efficacy of talampanel and its effect in modulating the AMPA-receptor (AMPAR) responses in neonatal seizures and brain injury. Dr. Jensen's and Dr. Fetell's research is based on a series of in vivo and in vitro models of neonatal seizures and perinatal injury demonstrating selective anticonvulsant, antiepileptogenic, and protective efficacy of known AMPA receptor antagonists NBQX, GYKI-52466, and topiramate. Their project aims to test neonatal and pediatric efficacy and toxicology as well as begin preliminary development of a non-oral form of talampanel that is easily administered to human infants.
- Project Title: Intracerebral Injection of Anticonvulsant Containing Spray-Dried Biocompatible Microparticle for Long-Term Treatment of Epilepsy
Bi-Botti Celestin Youan, PharmD., Ph.D., School of Pharmacy
Karin Borges, Ph.D.
Texas Tech University Health Sciences Center School of Pharmacy
Amarillo, Texas
Summary: The researchers were awarded a grant to explore the efficacy of spray-dried biocompatible microparticles of phenytoin, when injected into the brain, result in optimal therapeutic anticonvulsant concentrations and seizure control. Using phenytoin as the model drug, Dr. Youan's and Dr. Borges' project aims to engineer novel, safe and effective therapies for long-term treatment of epilepsies by providing the platform for the development of a delivery system capable of administering new and old anticonvulsant drugs. The long-term goal is to provide a treatment alternative for patients who are pharmacoresistant and not candidates for surgery.
April 2005: Epilepsy Therapy Project - Investment
- VistaGen Therapeutics, Inc. (www.vistagen-inc.com)
Burlingame, California
Summary: Founded in 1998, VistaGen Therapeutics, Inc. is a development stage biopharmaceutical company focused on developing and commercializing small molecule and protein therapeutics for central and peripheral nervous system ("neuronal") disorders and metabolic diseases (diabetes, obesity, and other insulin-resistance conditions). Since inception, the Company has achieved significant milestones, including: (i) assembling a strong patent position in embryonic stem cell ("ES cell") technologies; (ii) a strategic pharmaceutical ES cell drug development partnership in diabetes; and (iii) the acquisition of Artemis NeuroScience and its lead compound, AV-101.VistaGen expects to file an IND in 2006 and enter the clinic with AV-101 for multiple neuronal disorders in late 2006. VistaGen believes AV-101, it's lead drug candidate for the treatment of epilepsy and neuropathic pain, represents a potential first-in-class therapy in the growing, multi-billion dollar worldwide neuronal market. In addition, VistaGen has a proprietary ES cell-based drug discovery platform technology, with one significant partnership based on this core technology already in place. VistaGen expects to enter into additional partnerships to obtain new drug candidates, fund research and development, provide milestone-based funding, and market commercial products that result from collaborations.
April 2005: Epilepsy Therapy Project Grant Award
- Project Title: A Stereoselective Comparative Analysis of the Antiepileptic and Antiallodynic Activity and Lack of Hepatotoxicity of the Enantiomers of Propylispopropyl Acetamide (R-PID, S-PID and Racemic-PID), a Second Generation to Valproic Acid
Meir Bialer, Ph.D., MBA, David H. Eisenberg, Professor of Pharmacy
Boris Yagen, Ph.D., Professor of Medicinal Chemistry, School of Pharmacy, Faculty of Medicine
The Hebrew University of Jerusalem
Jerusalem, Israel
Summary: The researchers were awarded a grant to stereoselectively assess, utilizing animal modes, the antiepileptic and antiallodynic activity of propylisopropyl acetamide (R-PID, S-PID and racemic PID) a second generation to valproic acid (VPA). In an ongoing research aiming to develop more potent and less toxic antiepileptics and CNS drugs, utilizing pharmacokinetic-pharmacodynamic-relationship (SPPR) approach, a series of amide analogues of VPA has been designed and comparatively analyzed. PID is one of the lead compounds of this series and the project aims to enantioselectively assess the anticonvulsant and antiallodynic activity and lack of hepatotoxicity of PID (individual enantiomers and racemate) in animal models for epilepsy, neuropathic pain and VPA-associated hepatotoxicity.VPA is one of the leading antiepileptic drugs (AEDs). However its clinical use is restricted in women of child-bearing age and in children due to its teratogenicity and hepatotoxicity. Therefore, there is a substantial need to develop non-teratogenic and non-hepatotxic CNS-active VPA derivatives. The design and development of PID enantiomers can provide a suitable answer to this clinical need.
This grant from the Epilepsy Therapy Project was matched with an equal grant from Jazz Pharmaceuticals, Inc. (www.jazzpharmaceuticals.com), Palo Alto, California.
December 2004: New Therapy Grant Awards
- Project Title: Preclinical Evaluation of Ginseng Extract
Janet L. Stringer, M.D., Ph.D., Department of Pharmacology
Baylor College of Medicine
Houston, Texas
Summary: The researcher was awarded a grant to determine the effects of particular ginseng extracts, or naturally occurring compounds known as ginsenosides in the treatment of seizures. Dr. Stringer's project is based on a research finding that a specific extract produced from ginseng leaves has greater anticonvulsant activity than the whole ginseng extract, Dr. Stringer's program aims to identify and characterize anticonvulsant ginsenosides, as well as to purify and prepare sufficient quantities of the extract for future clinical studies.
- Project Title: Noninvasive Nonlinear Seizure Prediction Device
Robert Savit, Ph.D., Professor of Physics
Jonathan Edwards, M.D., Clinical Assistant Professor of Neurology, Director, EEG Laboratory
University of Michigan
Ann Arbor, Michigan
Summary: The researchers were awarded a grant to complete testing and produce definitive proof of a concept for an ambulatory non-invasive recording device capable of providing advanced notice of an impending seizure. In previous research, Dr. Savit and his collaborators had developed a measurement of marginal predictability (MP) for nonlinear time series. Under an R01 grant from the National Institutes of Health, Dr. Savit and his collaborators observed significant, consistent changes in MP several tens of minutes prior to a seizure in patients with focal epilepsy. Based on these findings, Dr. Savit and Dr. Edwards' project aims to test the efficacy of changes in MP as a predictor of impending seizures as well as determine if the algorithm used for prediction is universal across epilepsy patients. The ability to predict seizure onset could be the foundation for future intervention devices and therapies preventing seizures before they occur.
- Project Title: Controlling Brain Excitability by Electrical Stimulation
Idil Cavus, M.D., Ph.D., Assistant Professor, Psychiatry and Neurosurgery Departments
Yale University
New Haven, Connecticut
Summary: The researcher was awarded a grant to study the control of brain excitability by electrical stimulation in patients with epilepsy. Dr. Cavus' project is based on his preliminary findings that seizure suppression is correlated to the frequency of the stimulation: low frequency (1Hz) and high frequency (130-333 Hz) appear safe and efficacious; mid-range frequency (50 Hz) is not. Dr. Cavus aims to compare the effects of stimulating the epileptogenic neocortex of the brain at low and high frequencies on neurophysiological and neurochemical excitability measures in neurosurgical epilepsy patients. Currently available implantable stimulation devices are successful at treating other movement disorders, but have been ineffective at controlling many types of epilepsy seizures. The results will determine if frequency is a factor in efficacy and will serve as a guide for additional, comprehensive studies involving other stimulation sites, excitability measures, duration of stimulus, repetitive stimulation, in addition to other possible endpoints.
May 2004: Epilepsy Therapy Project Translational Research Grant Awards
- Project Title: Asian Herbs for Epilepsy
Steven Schachter, MD, Professor of Neurology
Harvard Medical School
Associate Director, Clinical Research
Harvard Medical School Osher Institute
Boston, Massachusetts
Summary: The researcher was awarded a grant to examine Asian herbs as a possible source of new chemical entities for the treatment of epilepsy. This research leverages an existing National Institutes of Health (NIH)-funded international collaboration in herbal research centered at Harvard Medical School, the National Institute of Neurological Disorders and Stroke (NINDS) Anticonvulsant Screening Program, and two high-throughput screening (HTS) laboratories at Harvard Medical School. The program also includes investigators at universities throughout Asia where a large number of herbs have been used to treat convulsive diseases. The study aims to evaluate promising herbal epilepsy medicines in highly validated animal models of epilepsy, and to identify, characterize, and profile the fractions and compounds with anti-seizure properties, and prioritize those herbs for future clinical studies.
- Project Title: Low Power Analog Seizure Detection Device
Naresh Bhavaraju, PhD, Research and Development Engineer
Flint Hills Scientific, LLC
Lawrence, Kansas
Summary: The recipient was awarded a grant towards the development of low-power, implantable seizure detection and treatment devices that will allow prolonged operation without the need for frequent battery recharging or replacement. The grant supports the development of an analog circuit implementation of FHS's seizure detection algorithm that reduces power consumption, prolonging battery life.
- Project Title: Animal Model of Posttraumatic Pharmacoresistant Epileptogenesis
Raimondo D'Ambrosio, PhD, Research Assistant Professor of Neurological Surgery
University of Washington School of Medicine
Seattle, Washington
Summary: The researcher was awarded a grant to establish baseline data on a new animal model of epileptogenesis and pharmacoresistant epilepsy. This project is designed to evaluate a rodent model with posttraumatic epilepsy via closed head injury-induced seizures for drug screening. This model is closely related to human cases of closed head injury. Its novel design was developed in response to increasing concern that traditional epilepsy models, which rely on electrical stimulation or administration of neurotoxins, may be clinically irrelevant and ineffective at identifying significant epileptogenic mechanisms and antiepileptic therapeutics. The project will focus on the probability of developing epilepsy due to the degree and location of injury, the neurologic focus of epilepsy following injury over time, and the pharmacological responsiveness at different durations of time following injury.
April 2004: Epilepsy Therapy Project - Investment
- Marinus Pharmaceuticals, Inc. (www.marinuspharma.com)
New Haven, Connecticut
Summary: Marinus is a development stage specialty pharmaceutical company dedicated to the discovery and development of drugs to treat serious neurological and psychiatric disorders. The company has successfully in-licensed a phase II/III compound for epilepsy as well as a late pre-clinical program for bipolar disorder from Yale University. In addition, the company has developed several proprietary high throughput assays of epilepsy. Fred Frank of Lehman Brothers is the lead investor. The company is led by Geoffrey E. Chaiken, President and Founder, and Harry H. Penner, Chairman and CEO.
March 2004: Epilepsy Therapy Project - Investment
- NeuroMolecular Pharmaceuticals, Inc. (www.neuromolecular.com)
Emeryville, California
Summary: NMI was founded in 2002, and its approach is to utilize NMDA receptor channel compounds to treat a variety of CNS disorders, including epilepsy. It is developing a class of neurprotective drugs based upon Memantine. NMI is headed by Gregory Went and includes Stuart Lipton and Jonathan Stamler as founders and co-chairs of its Scientific Advisory Board.
Resources
Epilepsy Centers
Epilepsy centers provide you with a team of specialists to help you diagnose your epilepsy and explore treatment options.
Epilepsy Medication
Find in-depth information on anti-seizure medications so you know what to ask your doctor.
Epilepsy and Seizures 24/7 Helpline
Call our Epilepsy and Seizures 24/7 Helpline and talk with an epilepsy information specialist or submit a question online.
Tools & Resources
Get information, tips, and more to help you manage your epilepsy.