What Is Clinical Research?
Epilepsy News From: Tuesday, January 24, 2017
In this article, Basic Science Editor Sloka Iyengar PhD answers questions about clinical research, including how its conducted and what steps are taken to keep participants' information safe and private. She also suggests questions you might ask if you are considering participating in a clinical trial. Learn more about clinical trials.
What is clinical research?
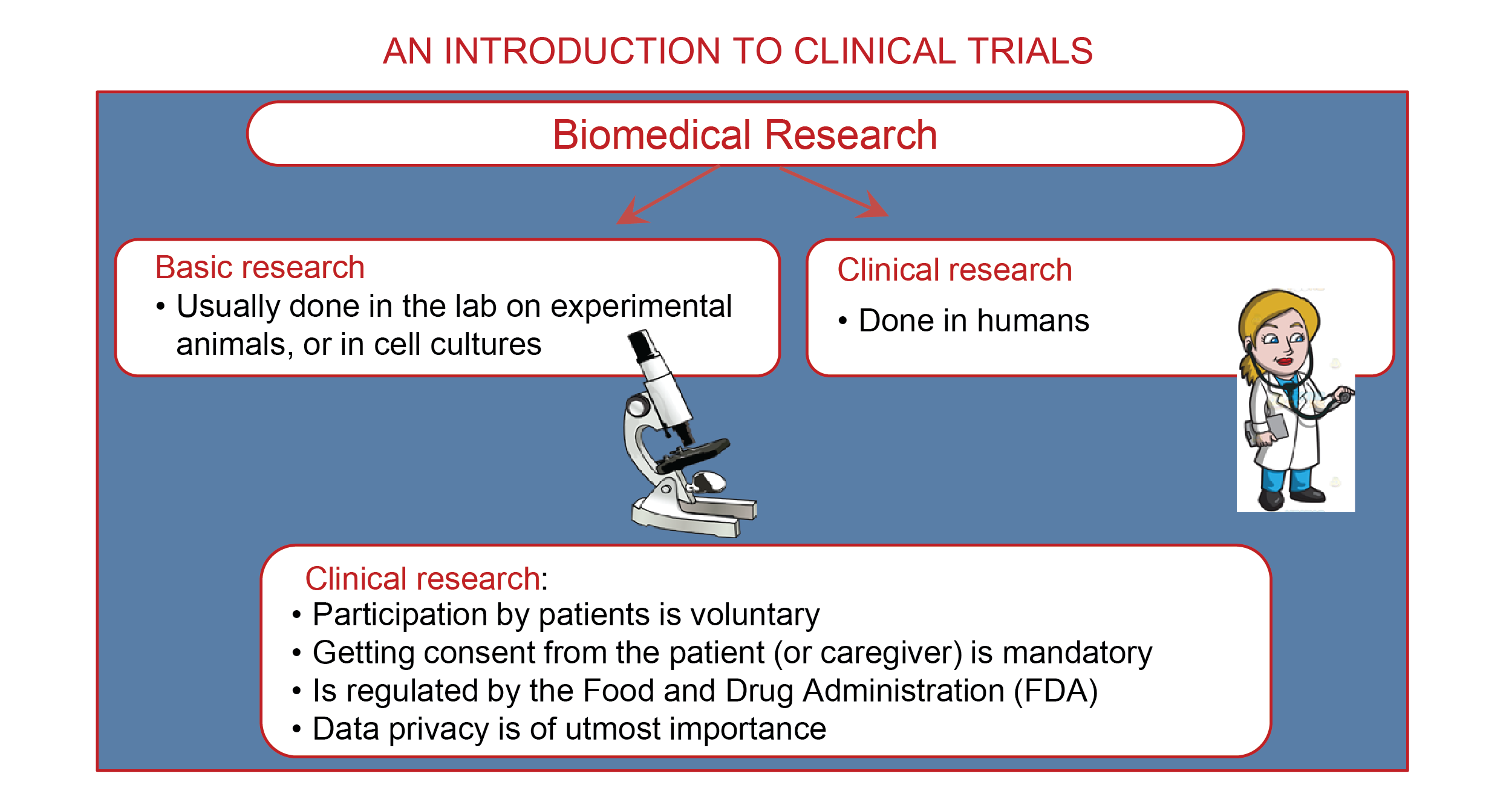
- Research in medicine can be of two kinds: basic or clinical.
- Basic research is usually done in the lab by people trained to examine specific questions using experimental rodents (rats and mice) or cell cultures. Read more about basic research in epilepsy.
- Clinical research involves human patients. It could be done to test new drugs or to look at patterns in data that has already been collected, called a “retrospective study.”
- Clinical research plays an important part in developing new therapies for people with neurological (or any) illness. Some diseases have very few effective treatment options; for these, clinical research is the only way we can ensure better therapies for people affected by the disease in the future.
What does clinical research entail?
- The process of bringing a new drug to people is long and expensive. Only one out of 5,000 to 10,000 potential new drugs is ultimately approved by the U.S. Food and Drug Administration (FDA) for use in the clinic.
- Each clinical trial has eligibility criteria that differ according to the type of study being done.
- All participants are willing volunteers who go through an extensive consent process to make sure they understand their role and any potential risks. Participants are free to end their participation in the study any time they wish.
- Before a clinical study is started, the clinicians or scientists involved are required to submit to a regulatory board a detailed plan that describes the study plan and goals. The regulatory board is called the Institutional Review Board (IRB). Study proposals are approved only if the board is convinced that the study is safe, ethical, and necessary.
What is data privacy, and what is being done to ensure information about me is kept safe?
- Data obtained as a result of clinical research is highly confidential. Hence, it is very important for clinicians and scientists to responsibly store and use this data. Once the data has been collected, reporting and publishing this data can be done only by qualified professionals.
- In addition to ensuring the study is conducted safely, regulatory boards also have strict conditions for data privacy. Only certain certified individuals have access to data. Depending on the type of study, care is taken to make sure that data is de-identified or made anonymous. This means the person’s name is replaced by a series of numbers; researchers never know the participant’s real name, only this number.
- The plan for the study has detailed descriptions of record keeping and data handling procedures. Each study participant has a case report form (CRF) that is kept by a certified professional in a safe, locked place. There are strict regulations about how and where the CRFs should be kept, what to do in case corrections need to be made to the CRFs, and steps to be taken in case the CRFs need to be transported. This important document contains information on everything related to a certain individual, such as medical history, treatments received, side-effects (if any), tests done, and results obtained.
What questions I should ask the doctor or scientist conducting the study?
Each trial and each person in it is unique. There may be special considerations for each trial and for each person participating. Understanding what will happen during the study is important. Here is a list of questions you might ask the doctor, scientist, or the study coordinator (the person at the hospital or the clinic who facilitates the clinical trial):
- What is the purpose of this study?
- How long do I (or my child / loved one) have to participate in this study?
- How often do I need to come to the clinic?
- Approximately how much time can I expect to spend per clinic visit?
- What tests and procedures will be done and how often?
- What are the benefits of participating in this study?
- What are the risks?
- Do I need to pay for any of this? Will I be compensated?
- Will the results from this study be published? If yes, will the publication be available to the public and can I be notified when it is available?
- What needs to be done if I decide to end my participation in this study?
Authored by
Sloka Iyengar PhD
Reviewed Date
Tuesday, January 24, 2017
