Tiagabine Hydrochloride
Gabitril (GAB-ih-tril) is the brand name for the seizure medicine tiagabine (tie-AG-ah-bean) hydrochloride (HI-droh-KLOR-ide), or just tiagabine. Gabitril is available in the United States, the United Kingdom, and Australia, but not in Canada. Gabitril is not available in generic (non–brand name) form in the United States.
Gabitril is marketed in the United States by Cephalon, Inc. The name or appearance may differ in other countries. The dose (measured in milligrams, abbreviated "mg") will usually be the same. These descriptions apply to the U.S. versions.
Updated: 16/10/2023
Brand Name(s)
Gabitril
Used to Treat
Forms

2-mg (orange-peach color, round)
Tablet with "C" logo on one side and "402" on the other
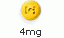
4-mg (yellow, round)
Tablet with "C" logo on one side and "404" on the other

12-mg (green, ovaloid)
Tablet with "C" logo on one side and "412" on the other

16-mg (blue, ovaloid)
Tablet with "C" logo on one side and "416" on the other
Package Insert
Frequently Asked Questions
How to take and store Tiagabine Hydrochloride?
Follow your doctor's directions. Call if you have any questions. Usually, your doctor will tell you to start by taking one tablet – either 2 or 4 milligrams – two times each day. After a while, the doctor may gradually increase the dosage to get better control of your seizures. Because Gabitril (tiagabine) is generally taken up to four times a day, many people help themselves to remember by taking it with meals and at bedtime. It should be taken with food. Ask the doctor what to do if you forget to take a dose.
Be careful if the doctor writes a new prescription using a different kind of pill. For example, if you've been using 4-mg tablets and the new prescription is for 12-mg tablets, be careful to use the correct number. Don't automatically continue to use the same number of pills as before. Make sure you know what size tablet has been prescribed.
All forms of Gabitril should be stored at room temperature, away from light and humidity. (Don't keep the pills in the bathroom if it's damp there.) Of course, keep all Gabitril out of the reach of children.
Don't take more than the doctor prescribes. Be sure to use only the amount of Gabitril that your doctor prescribes. If you think you've taken one or two extra tablets, call your doctor for advice. For a larger overdose, call your local poison control center or emergency room right away, unless you have special directions from the doctor.
Don't stop taking Gabitril or change the amount you take without talking to your doctor first. Stopping any seizure medicine all at once can cause serious problems.
What if I forget?
If you forget a dose, take it as soon as you remember. If it is almost time for the next dose, delay that dose for a few hours instead of taking two doses very close together. Then go back to the regular schedule. If you're not sure about what to do, call the doctor's office for more advice.
Do your best to follow the doctor's directions. If you forget doses often, it may be a good idea to get a special pillbox or watch with an alarm to remind you.
Taking the right amount of seizure medicine on time every single day is the most important step in preventing seizures!
How does Tiagabine Hydrochloride affect the brain?
Brain cells need to work (fire) at a certain rate to function normally. During a seizure, brain cells are forced to work much more rapidly than normal. Gabitril helps prevent brain cells from working as fast as a seizure requires them to. In this way, seizures can be stopped when they are just beginning.
How does the body digest Tiagabine Hydrochloride?
After medicine is swallowed, it must be absorbed into the blood so it can move throughout the body. The process of absorbing, digesting, and excreting a medicine or food is called metabolism. The way the body metabolizes a particular medicine affects how often it must be taken. It also determines whether it will interact with other medicines or be affected by liver disease or kidney disease.
Gabitril is metabolized by the liver. This process happens more quickly if certain other seizure medicines are also being used. The amount of Gabitril taken may need to be increased if you also take one of these other seizure medicines:
- carbamazepine (Tegretol, Tegretol XR, Carbatrol)
- phenytoin (Dilantin, Phenytek)
- phenobarbital
- primidone (Mysoline)
Because Gabitril is metabolized in the liver, people with liver disease need to be cautious. They may have to start with a lower dose and have their dosage increased more slowly than other people the same age.
How well does the Tiagabine Hydrochloride work?
Doctors have studied large numbers of people with partial seizures to find out how well Gabitril works. Studies have shown that Gabitril works well when added to other seizure medications. Gabitril is not a perfect add-on seizure medicine for everyone, however. Sometimes people must try a series of combinations before finding what is best for them.
Some other medications affect how Gabitril is eliminated from the body, so the dosages may need to be changed with different combinations.
What are the most common side effects of Tiagabine Hydrochloride?
Many people who take Gabitril (tiagabine) don't report any unwanted side effects. Those who experience undesirable effects most often complain of:
- dizziness
- tiredness
- nervousness
- sleepiness
- difficulty concentrating
- tremor
Be careful when you first start taking Gabitril. Make sure you don't have a problem with sleepiness or dizziness when driving or doing anything else that might be dangerous.
Some other side effects mentioned even less often were:
- nausea
- vomiting
- diarrhea
- weakness (for example, sometimes people say that their knees buckled)
- irritability
- confusion
If you notice any of these problems, call the doctor's office. Sometimes the doctor can help by changing the amount of Gabitril you take or how you take it. Don't stop taking Gabitril or change the amount you take without the doctor's guidance.
Some of these problems become more severe when higher doses of Gabitril are taken, or when the dosage is increased rapidly.
Be sure to read about the serious side effects so you will be aware of a few serious problems that could arise when you take Gabitril. These serious problems are very rare but everyone who takes this medicine should at least be aware of them.
Allergic reactions
There do not appear to be any allergic reactions to Gabitril.
Long-term side effects
There are no known long-term side effects of Gabitril.
What are the most serious side effects of Tiagabine Hydrochloride?
Very few people have serious reactions to Gabitril. If you take it, you should be aware of them, however, so you and your family can recognize them.
About 1 in 100 people who have taken Gabitril have experienced a lot of weakness. In every case, the weakness went away after the Gabitril was reduced or discontinued.
In rare cases, Gabitril causes severe confusion. It is a medical emergency and is usually treated with clonazepam (Klonopin) or lorazepam (Ativan). People with a history of absence seizures or with a type of EEG abnormality called “spike-wave” appear to be at particular risk.
On July 10, 2008, an advisory panel was convened by the Food and Drug Administration (FDA) to review data that the FDA had previously collected from drug studies showing an association between many of the antiepileptic drugs (AEDs) and suicidal ideation and behavior, which together are called suicidality. According to the FDA’s Alert, among the patients with epilepsy in these drug studies, 1 out of 1000 people taking the placebo (inactive substance) showed suicidality compared to approximately 3.5 out of 1000 people who took an AED. The FDA advisory panel voted to accept the FDA's data at its meeting on July 10.
- Taking antiepileptic medicines may increase the risk of having suicidal thoughts or actions;
- Do not make any changes to the medication regimen without first talking with the responsible healthcare professional;
- Pay close attention to any day-to-day changes in mood, behavior and actions. These changes can happen very quickly so it is important to be mindful of any sudden differences.
- Be aware of common warning signs that might be a signal for risk of suicide. Some of these are:
- Talking or thinking about wanting to hurt yourself or end your life
- Withdrawing from friends and family
- Becoming depressed or having your depression get worse
- Becoming preoccupied with death and dying
- Giving away prized possessions
We again urge patients and families to contact their doctor before stopping an epilepsy medication because this may possibly lead to seizures and worsening of mood.
Impact of Tiagabine Hydrochloride on bone health
See package insert.
What else is Tiagabine Hydrochloride used for?
Often doctors find that medicines are useful for more than one purpose. It is legal to prescribe medicines for ""off-label uses"" even though the FDA has not formally approved such use. Off-label uses of Gabitril include:
- spasticity
- migraine headaches
- mood disorders
- other seizure types such as infantile spasms
Cephalon Inc., the manufacturer of Gabitril, issued health care providers a letter in February 05 informing them that the Food and Drug Administration (FDA) will add a bolded warning to the label of Gabitril (tiagabine hydrochloride) to warn of the seizure risk associated with the drug when it is used off-label to treat patients without epilepsy.
Who should not take Tiagabine Hydrochloride?
The only people who definitely should not take Gabitril are those who are allergic to it or any of its inactive ingredients.
Also, people with a diagnosis of absence seizures or an EEG abnormality called “spike-wave" should not take Gabitril.
People who have liver disease need to be extra cautious about taking Gabitril.
Can Tiagabine Hydrochloride be taken with other medicines?
Sometimes one kind of medicine changes the way another kind of medicine works in the body. If two kinds of medicine affect each other, the doctor may prescribe something else or change the amount to be taken.
Your body gets rid of Gabitril quicker if you are also taking certain seizure medicines, such as:
- carbamazepine (Tegretol, Tegretol XR, Carbatrol)
- phenobarbital
- primidone (Mysoline)
- phenytoin (Dilantin, Phenytek)
If this applies to you, your doctor may plan to give you more Gabitril than you would otherwise take.
Any time one of your doctors suggests starting or stopping a prescription, be sure to ask what effect it might have on the Gabitril, and whether the dosage should be increased or decreased.
Gabitril has no known effect on other medicines that you may be taking.
What are the effects of Tiagabine Hydrochloride on Children?
Gabitril is used to treat children with partial seizures, and also has been used to treat infantile spasms (West syndrome), although it is not approved by the Food and Drug Administration (FDA) for this purpose.
Doctors figure out how much medicine to give to young children based mostly on their weight. To keep side effects at a minimum, the doctor probably will prescribe a low dose to start with and increase it slowly. Children usually start with a dose of 0.1 (one-tenth) milligram (mg) for each kilogram (kg, about 2.2 pounds) of their body weight per day. This is usually given in 2 or 3 equal doses.
Most children do best at about 0.1 to 2 mg per kg per day, which may require gradually increasing the dose as directed by the doctor.
If a woman takes Tiagabine Hydrochloride during pregnancy will it hurt the baby?
In the United States, the Food and Drug Administration (FDA) assigns each medication to a Pregnancy Category according to whether it has been proven to be harmful in pregnancy. Gabitril is listed in Pregnancy Category C. This means that caution is advised, but the benefits of the medicine may outweigh the potential risks. Studies in animals have shown some harm to the baby, but there haven't been any good studies of results in women.
Talk to your doctor or another health professional if you are pregnant or plan to become pregnant. We don't yet have enough information to be able to estimate the risk of various types of birth defects that might occur if Gabitril is taken during pregnancy. We also don't know enough to compare the risk with Gabitril to the risk with other seizure medicines. The risk of birth defects is generally higher for women who take more than one seizure medication and for women with a family history of birth defects.
Women who are capable of becoming pregnant should take at least 400 mcg (0.4 mg) of folic acid (folate) daily to help prevent a type of birth defect called a neural tube defect. (The best-known of these is spina bifida, in which the spinal cord is not completely enclosed.) Women at high risk, such as those who have already had a baby with this kind of defect, should take 4000 mcg (4 mg) daily, beginning before they become pregnant.
How much Gabitril is passed through breast milk is not known for certain, but the way the body uses it suggests that probably a large portion does enter the milk. If you want to breastfeed your baby, check with your doctor about what seizure medicine would be best for you.
What are the effects of Tiagabine Hydrochloride on Seniors
Gabitril is commonly prescribed for people over 65, but it's important for the doctor to be careful about how much Gabitril these people take, because seniors may be more sensitive to the side effects of Gabitril, such as dizziness and tremor. Also, seniors are at greater risk of injury from falls or other accidents.
What are the dose ranges for Tiagabine Hydrochloride?
The best amount is the amount that completely controls seizures without causing troublesome side effects. It depends on many factors, which are different for every individual. Follow the doctor's directions. Call if you have any questions.
In young adults, Gabitril is usually started at 4 milligrams (mg) per day, and increased by 8 to 12 mg per month as required, to a maximum of 32 to 56 mg per day, if side effects are not troublesome.
People over age 65 generally require a lower dose and longer period between increases.
No one should stop taking Gabitril or change the amount they take without talking to the doctor first. Stopping any seizure medicine all at once can cause a problem that may be life-threatening.
Don’t use more than the doctor prescribes. If a little extra (such as an extra tablet or two) is taken by accident, call the doctor for advice. For a larger overdose, call a poison control center or emergency room right away unless you have other specific directions from your doctor.
Those who have been taking another seizure medicine may be told to continue to take it in the same way as before, or the amount of the other medicine may gradually be reduced over several weeks to months.
Read the package insert of Tiagabine Hydrochloride
In the United States, companies that manufacture medicines are required to publish certain kinds of information about each product. This document is commonly known as a “package insert” because it is usually included with each package of the medicine.
You can also read these documents (also called "prescribing information") online. The U.S. package insert for Gabitril (tiagabine) is found at:
Some of the information may differ in other countries.
To learn how to read and understand a package insert, see "How to read a package insert."

Primary Generalized Epilepsy
