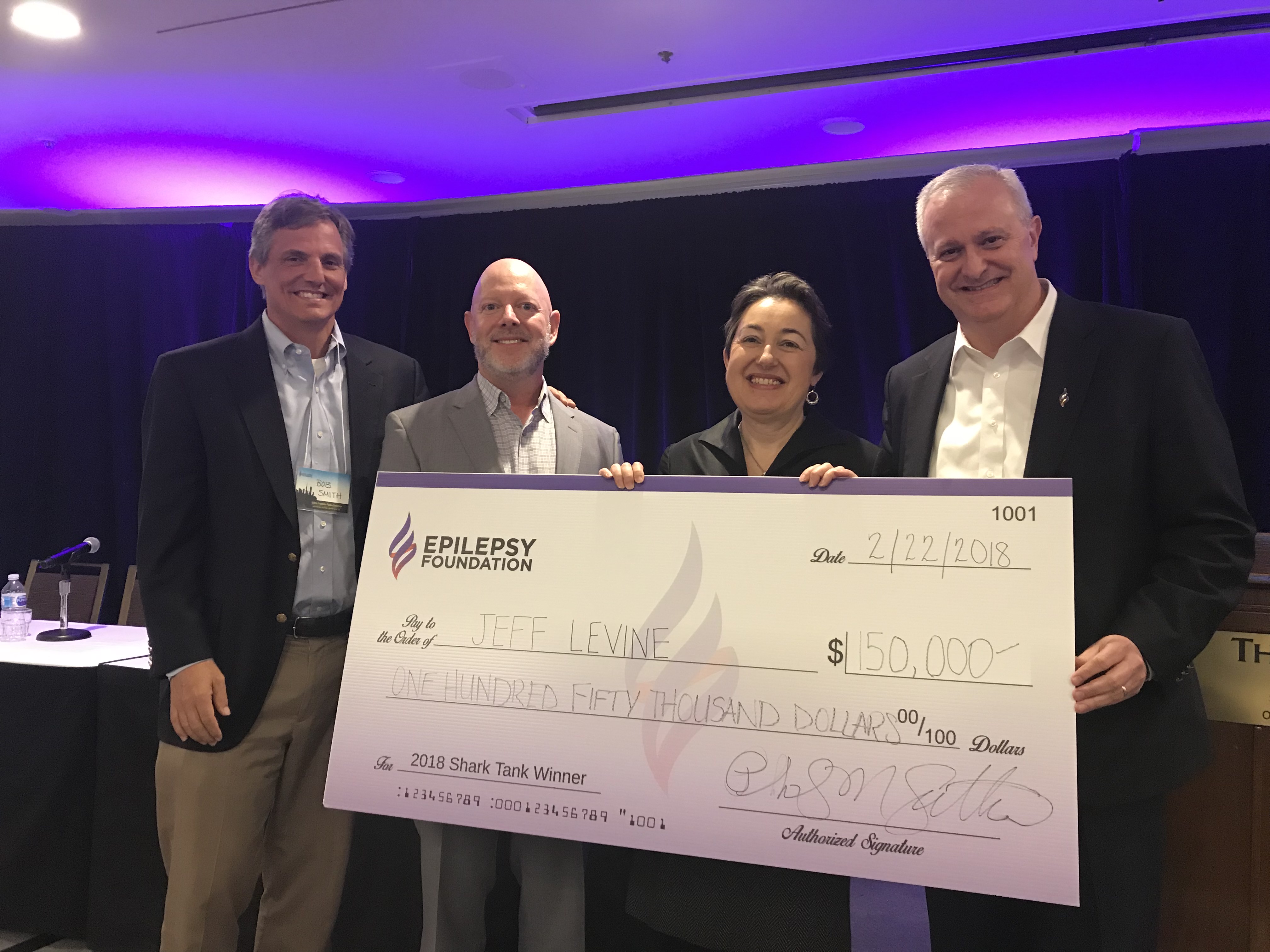
Shark Tank Competition Winners

The Epilepsy Foundation’s Shark Tank Competition seeks to advance innovative ideas in epilepsy and seizure treatment and care. The winners will use their prize to accelerate their innovation to the next phase of development and closer to being accessible to people living with epilepsy.
In total, 26 individuals or teams entered the competition from across the United States and 9 countries throughout the world. From this pool, 6 teams were chosen as finalists. The finalists presented at the 2018 Epilepsy Foundation Pipeline Conference on February 22, 2018, in San Francisco, California.
2018 Shark Tank Competition Winners
3D Machine Vision System for Surgical Navigation of the Human Brain
Aaron Bernstein PhD, President and CEO, Advanced Scanners
Jeff Levine MIM, Advanced Scanners
- 3D vision platform to reduce the uncertainties and risks of brain surgery
- Project aims to track brain shift in real time to reduce navigational errors for surgeons
Read More...
Open brain surgery begins with the surgeon creating an opening in the skull. The surgeons rely on image-guided navigation systems to track their movements within the brain. These navigation systems rely on previously scanned images of the patient’s brain. The problem is that when you open the skull, the brain changes its shape. Advanced Scanners wants to improve navigational systems and surgical outcomes with a rapid, noninvasive 3D scanning approach. Working with neurologists and neurosurgeons at the Dell Children’s Comprehensive Center in Austin, Texas, Advanced Scanners has been developing an intelligent 3D machine vision system. This system inter-operatively watches and tracks the exposed brain with sub-millimeter accuracy to improve results in each of the 3 major steps of a typical 2-stage epilepsy surgery. These steps include providing accurate location of the grid electrodes, mapping at high resolution the surface of the brain during surgery to improve what the neurosurgeon sees and updating the brain shape changes during real time. This all contributes to a more precise navigation system for the surgeon to rely on. This also should make the brain surgery procedure safer for the patient.
$50,000 Judges Award

Virtual Reality Medical Simulation for the Management of Status Epilepticus
Joshua M. Sherman MD, Faculty Attending – Division of Emergency Medicine, Children’s Hospital Los Angeles
Todd Chang MD, MACM, Assistant Professor of Pediatrics, USC-Keck School of Medicine
- This project is an immersive virtual reality module that would train health professionals in an emergency, like status epilepticus, at a lower cost.
- This training would be portable and standardized while remaining immersive.
- Drs. Sherman and Chang will use Shark Tank funds to create virtual reality simulations for other epilepsy scenarios.
Read More...
Drs. Sherman and Chang want to develop virtual reality simulations to allow the community to better train both medical and non-medical professionals for high emergency epilepsy situations. Usually trainings are done using mannequins and actors, which are expensive and constrained to when the actors are available. In contrast, a virtual reality (VR) module is portable, standardized, and still allows for an immersive experience. VR allows for training at any time of day. The doctors had partnered up with Oculus’ VR for Good program and companies AiSolve and Bioflight to design a module for status epilepticus in the pediatric population. This simulation won Best Virtual Reality Education Project at the 2018 VR Fest and was written up in USA Today and Buzzfeed. Using an Occulus Simulator, trainees can be assessed on their training readiness and taught what to do in high-stakes situations like status epilepticus.
Shark Tank Finalists
Finalists were awarded a $5,000 prize.
Automated Detection of Epileptic Lesions from MRI
Nick Schmansky, Co-Founder and CEO, Cortico Metrics, LLC
Emily Lindemer, Staff Scientist, CorticoMetrics, LLC
- This team developed a software-based tool that can detect focal brain lesions, which can be tied to seizure activity, on an MRI scan, as well as other related brain abnormalities.
- This project will pilot this tool in epilepsy clinics across the United States. The pilot will look at patient outcomes, as well as look at the user experience for clinicians.
Machine Vision Based Solution for Noninvasive Seizure Quantification and Alarm
Kaapa Annala, CEO, NeuroEventLabs
- NeuroEventLabs aims to provide reliable data for improved treatment of epilepsy through a home monitoring system that captures seizure activity. This system would also serve as an alarm for caregivers.
- To identify seizures, the home monitoring system will look at breathing rhythm changes, seizure sound detection, and other elements.
Repositioning Compounds for Use in Refractory Epilepsy
John Somoza PhD, Senior Research Scientist, Gilead Sciences
- This project will identify compounds that could be used for treatment of epilepsy.
- To accomplish this, this team will build a network of individuals to speed up the identification and development of potential therapies. So far, there have been 4 compounds identified that could be effective in treating epilepsy.
Korwave
Patrick McFarland, Co-Founder and CEO, Korwave
- Korwave is a mobile brain monitoring device for people living with epilepsy. Worn on the head, it is designed to mimic the look of a Nike’s headband or headphones.
- The device will notify caregivers when a seizure occurs. Medical providers will be able to access seizure activity recorded by Korwave on a web platform.
Shark Tank Judges
- Congressman Tony Coelho
- Robert Fisher MD, PhD, Professor, Department of Neurology, Stanford University Medical Center
- Daniel Friedman MD, Associate Professor, Department of Neurology, N.Y.U. Langone Medical Center
- Elizabeth Garofalo MD, Principal, EAG Pharma Consulting
- Shivkumar Sabesan PhD, Staff Hardware Engineer, Verily Life Sciences LLC
Resources
Epilepsy Centers
Epilepsy centers provide you with a team of specialists to help you diagnose your epilepsy and explore treatment options.
Epilepsy Medication
Find in-depth information on anti-seizure medications so you know what to ask your doctor.
Epilepsy and Seizures 24/7 Helpline
Call our Epilepsy and Seizures 24/7 Helpline and talk with an epilepsy information specialist or submit a question online.
Tools & Resources
Get information, tips, and more to help you manage your epilepsy.